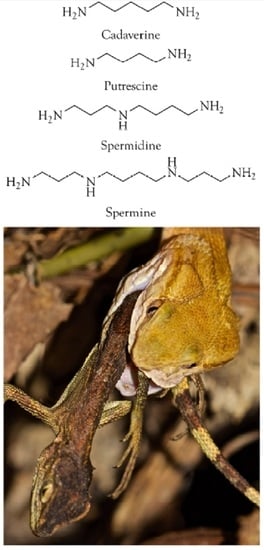
Polyamines as Snake Toxins and Their Probable Pharmacological Functions in Envenomation
Abstract
:1. Introduction
2. Results
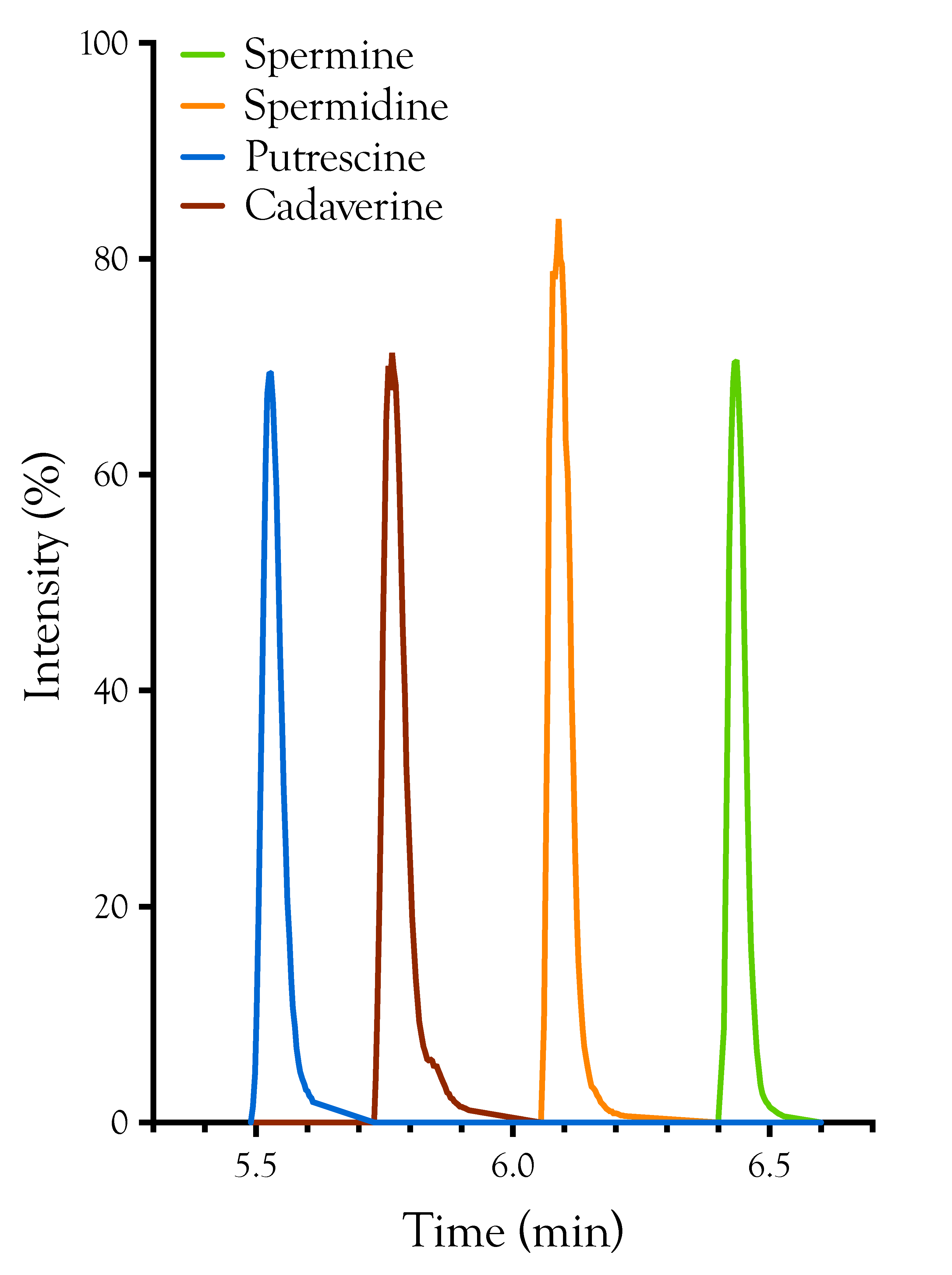
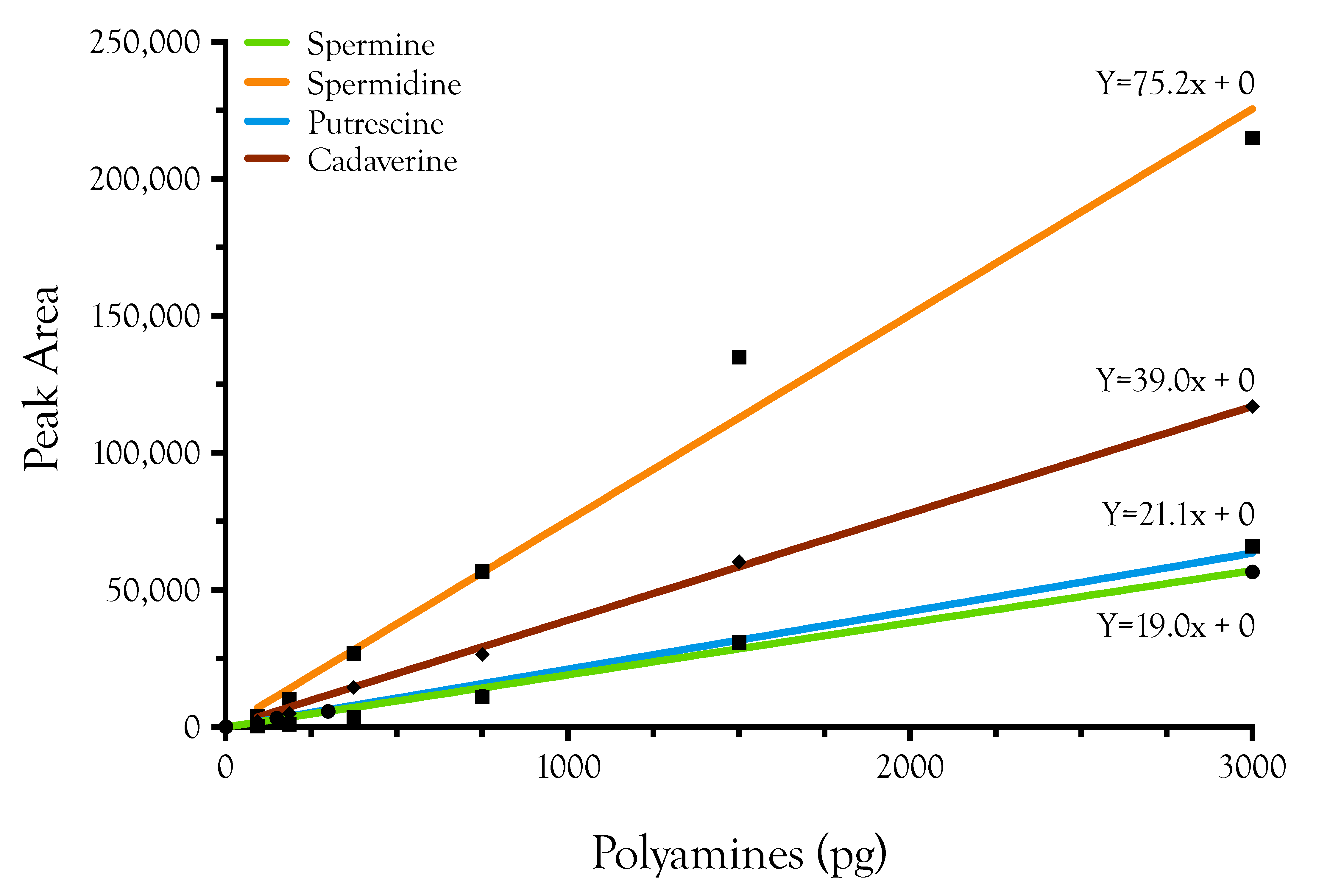
2.1. Detection, Quantification, and Distribution of Polyamines in Snake Venoms
2.2. Biological Significance of Snake Venom Spermine
Quantities of Polyamines Potentially Injected
2.3. Pharmacology of Spermine that Is Potentially Pertinent to Envenomation
2.3.1. Spermine Promotes Hypotension
2.3.2. Spermine Blocks L-Type Voltage-Dependent Calcium Channels, but Exerts Biphasic Effects on N-Type Channels
2.3.3. Spermine and Calcium Release (Ryanodine) Receptors
2.3.4. Spermine Potentiates NMDA Receptors (NMDARs) at µM Concentrations, but Inhibits Them at mM Concentrations
2.3.5. Spermine Blocks Type II AMPA Receptors (AMPARs)
2.3.6. Spermine and Kainate Receptors
2.3.7. Spermine Is a Nicotinic Acetylcholine Receptor Antagonist at µM Concentrations, but an Agonist at nM Concentrations
2.3.8. Spermine and Muscarinic Acetylcholine Receptors
2.3.9. Spermine Interacts with GABAA Receptors
2.3.10. Spermine Sensitizes Acid-Sensing Ion Channels
2.3.11. Spermine Sensitizes Capsaicin Receptors
2.3.12. Spermine Inhibits Platelet Aggregation
2.3.13. Spermine Inhibits Ca2+-ATPase
2.3.14. Spermine Promotes Breakdown of the Blood–Brain Barrier
2.3.15. Pharmacology of Spermidine, Putrescine, and Cadaverine that Is Potentially Pertinent to Envenomation
3. Conclusions
4. Materials and Methods
4.1. Venom Samples
4.2. Polyamine Derivatization
4.3. Liquid Chromatography/Mass Spectrometry of Polyamine Standards
4.4. Data Processing
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
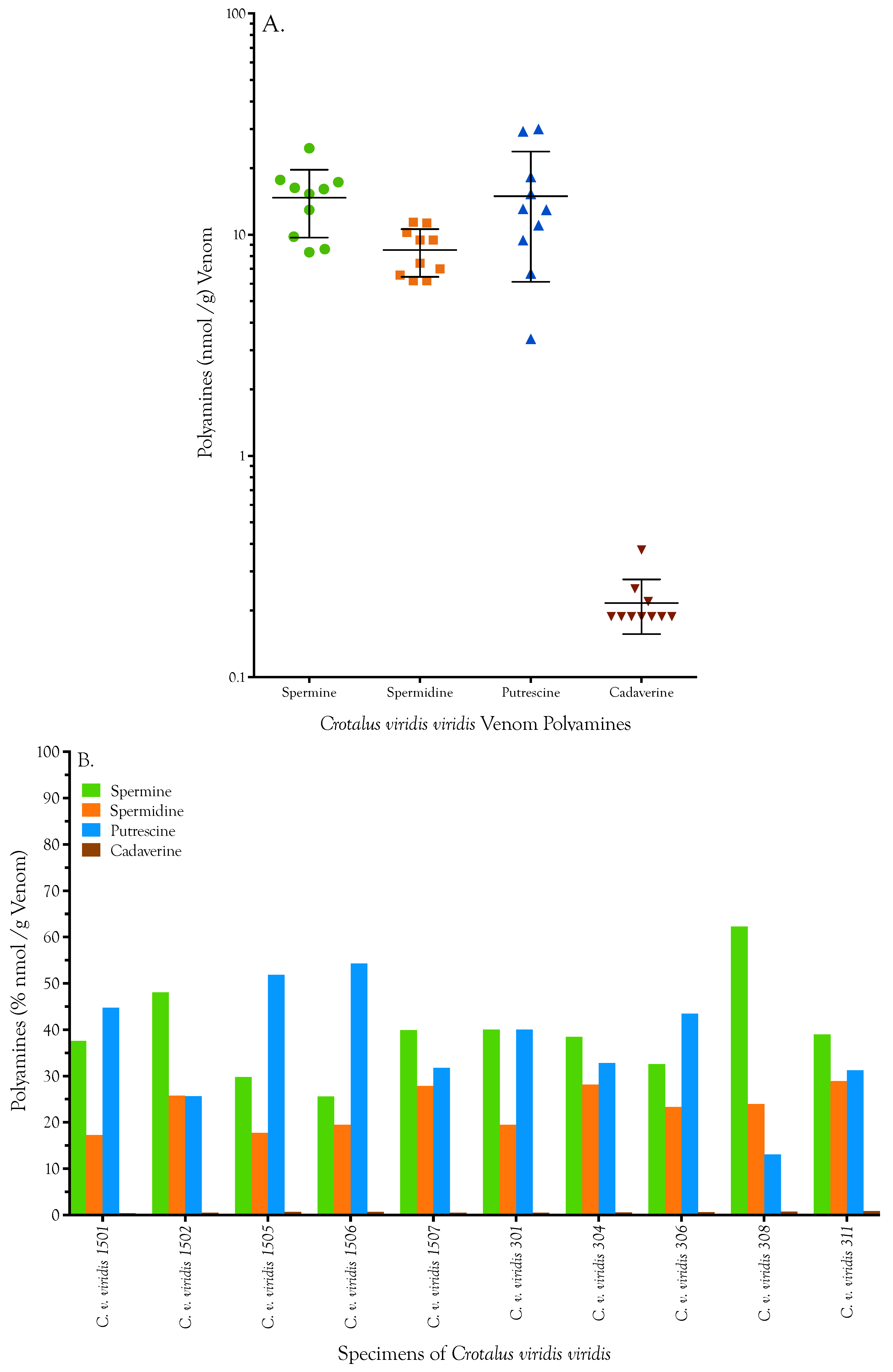
| Specimen | Spermine [nmol/g] | Spermidine [nmol/g] | Putrescine [nmol/g] | Cadaverine [nmol/g] | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| C. v. viridis 1501 | 24.640 | 24.576 | 11.280 | 11.324 | 28.896 | 29.695 | 0.251 | 0.251 | 65.456 |
| C. v. viridis 1502 | 17.776 | 17.586 | 9.561 | 9.385 | 9.511 | 9.366 | 0.188 | 0.188 | 36.781 |
| C. v. viridis 1505 | 17.555 | 16.985 | 10.355 | 10.134 | 29.913 | 30.130 | 0.376 | 0.376 | 57.912 |
| C. v. viridis 1506 | 8.761 | 8.445 | 6.565 | 6.565 | 18.296 | 18.223 | 0.188 | 0.251 | 33.648 |
| C. v. viridis 1507 | 15.277 | 17.302 | 11.015 | 11.720 | 12.996 | 12.923 | 0.188 | 0.188 | 40.805 |
| C. v. viridis 301 | 14.993 | 15.562 | 7.182 | 7.667 | 15.174 | 15.392 | 0.188 | 0.188 | 38.173 |
| C. v. viridis 304 | 12.873 | 13.000 | 9.694 | 9.253 | 10.745 | 11.326 | 0.188 | 0.188 | 33.634 |
| C. v. viridis 306 | 9.932 | 9.647 | 7.050 | 6.962 | 13.359 | 12.778 | 0.188 | 0.188 | 30.052 |
| C. v. viridis 308 | 16.036 | 16.131 | 6.213 | 6.169 | 3.412 | 3.340 | 0.188 | 0.188 | 25.838 |
| C. v. viridis 311 | 8.287 | 8.382 | 6.257 | 6.125 | 6.680 | 6.680 | 0.188 | 0.188 | 21.393 |
| Mean | 14.613 | 14.762 | 8.517 | 8.530 | 14.898 | 14.985 | 0.213 | 0.219 | 38.369 |
| Std. Deviation | 4.955 | 5.034 | 2.052 | 2.114 | 8.722 | 8.914 | 0.061 | 0.061 | 13.676 |
| Taxon | Polyamines (nmol/g venom) | Prey Species | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spermine | Spermidine | Putrescine | Cadaverine | Arthropods | Fish | Birds | Lizards | Anurans | Snakes | Mammals | ||
| Elapidae | ||||||||||||
| B. multicinctus | 0.032 | 0.000 | 0.944 | 0.376 | - | ❋ | - | - | - | ❋ | - | Mao, 1970 [152] |
| D. polylepis | 0.032 | 0.000 | 0.726 | 0.125 | - | - | ❋ | - | - | - | ❋ | Branch et al., 1995 [153] |
| M. surinamensis | 0.032 | 0.000 | 0.726 | 0.125 | - | ❋ | - | - | - | - | - | Olamendi-Portugal et al., 2008 [154]; Morais et al., 2011 [155] |
| N. kaouthia | 0.032 | 0.000 | 0.726 | 0.125 | - | - | - | - | - | ❋ | ❋ | Chanhome et al., 2001 [156]; Modal et al., 2016 [157] |
| N. sputatrix | 0.032 | 0.000 | 0.726 | 0.125 | - | - | - | - | - | ❋ | ❋ | Inferred |
| O. hannah | 0.063 | 0.000 | 0.871 | 0.188 | - | - | - | ❋ | - | ❋ | ❋ | Chanhome et al., 2001 [156] |
| Mean | 0.037 | 0.000 | 0.787 | 0.177 | ||||||||
| Std. Deviation | 0.013 | 0.000 | 0.097 | 0.100 | ||||||||
| Viperidae | ||||||||||||
| B. gabonica | 5.820 | 1.146 | 34.995 | 1.628 | - | - | ❋ | ❋ | ❋ | - | ❋ | Luiselli & Akani, 2003 [158] |
| B. nasicornis | 401.099 | 21.370 | 145.933 | 15.345 | - | - | - | - | ❋ | - | ❋ | Luiselli & Akani, 2003 [158] |
| C. cerastes | 1218.132 | 68.649 | 431.120 | 28.373 | - | - | - | ❋ | - | - | - | Bazaa et al., 2005 [159] |
| D. palestinae | 14.012 | 1.102 | 71.006 | 14.719 | - | - | - | - | - | - | ❋ | Inferred |
| D.siamensis | 251.142 | 14.320 | 406.870 | 15.220 | - | - | - | ❋ | ❋ | ❋ | ❋ | Chanhome et al., 2001 [156]; Gennaro et al., 2007 [160] |
| P. fieldi | 0.095 | 0.000 | 1.162 | 0.125 | - | - | ❋ | ❋ | - | - | ❋ | Ali et al., 2015 [161] |
| Mean | 315.050 | 17.764 | 181.848 | 12.569 | ||||||||
| Std. Deviation | 471.697 | 26.395 | 190.032 | 10.428 | ||||||||
| Crotalidae | ||||||||||||
| A. c. contortrix | 1087.374 | 3.084 | 72.386 | 14.907 | ❋ | - | ❋ | ❋ | ❋ | ❋ | ❋ | Trauth & McAllister, 1995 [162]; Heatwole et al., 1999 [163] |
| A. p. leucostoma | 398.474 | 4.318 | 14.521 | 3.006 | - | ❋ | - | - | ❋ | ❋ | ❋ | Himes, 2003 [164]; Vincent et al., 2004 [165] |
| A. nummifer | 0.253 | 0.044 | 0.871 | 0.125 | - | - | - | - | - | - | ❋ | Martins et al., 2002 [166] |
| B. schlegelii | 0.285 | 0.044 | 0.726 | 0.125 | - | - | ❋ | ❋ | ❋ | - | ❋ | Greene, 1988 [167]; Sorrel, 2007 [168] |
| B. erythromelas | 3.005 | 0.573 | 5.590 | 1.002 | ❋ | - | ❋ | - | ❋ | - | ❋ | Martins et al, 2002 [166] |
| B. moojeni | 516.295 | 10.090 | 72.313 | 14.719 | ❋ | - | ❋ | ❋ | ❋ | ❋ | ❋ | Nogueira et al., 2003 [169] |
| C. rhodostoma | 335.878 | 1.057 | 70.643 | 14.656 | ❋ | ❋ | ❋ | ❋ | ❋ | ❋ | ❋ | Daltry et al., 1998 [156]; Chanhome et al., 2001 [170] |
| C. sasai | 0.791 | 0.044 | 0.871 | 0.125 | ❋ | - | - | ❋ | ❋ | - | ❋ | Luiselli, 2006 [166]; Martins et al., 2002 [171] |
| C. adamanteus | 53.486 | 18.506 | 132.864 | 15.345 | - | - | - | - | - | - | ❋ | Margres et al., 2015 [172] |
| C. cerastes | 19.199 | 14.761 | 90.464 | 14.782 | - | - | - | ❋ | - | - | ❋ | Secure & Nagy, 1994 [173,174] |
| C. d. terrificus | 151.223 | 88.741 | 269.141 | 16.348 | - | - | - | - | - | - | ❋ | Salomao et al, 1995 [160]; Sant’anna & Abe, 2007 [175]; Gennaro et al., 2007 [176] |
| C. m. pyrrhus | 0.569 | 0.088 | 1.379 | 0.125 | - | - | - | - | - | - | ❋ | Meik et al., 2012 [177] |
| C.v.concolor | 9.805 | 7.314 | 13.795 | 0.188 | - | - | - | ❋ | - | - | ❋ | Mackessy et al., 2003 [178] |
| C. v. viridis | 14.687 | 8.524 | 14.942 | 0.216 | - | - | ❋ | ❋ | - | - | ❋ | Hayes, 1992 [179] |
| L. stenophrys | 129.683 | 6.213 | 22.943 | 2.944 | - | - | - | - | - | - | ❋ | Voss, 2013 [180] |
| O. okinavensis | 0.443 | 0.044 | 0.799 | 0.188 | - | - | ❋ | ❋ | ❋ | - | ❋ | Mori & Toda, 2011 [181] |
| P. elegans | 2320.150 | 1.234 | 70.498 | 14.719 | - | - | - | ❋ | - | - | ❋ | SDA, pers. observations |
| P. flavoviridis | 85.875 | 1.190 | 70.425 | 14.656 | - | - | ❋ | - | ❋ | - | ❋ | Gennaro et al., 2007 [160]; Chijiwo et al., 2003 [182] |
| P. mucrosquamatus | 7918.426 | 77.902 | 700.479 | 146.440 | - | - | ❋ | - | ❋ | ❋ | ❋ | Mao, 1970 [152] |
| Mean | 686.626 | 12.830 | 85.561 | 14.454 | ||||||||
| Std. Deviation | 1938.355 | 25.468 | 162.691 | 32.745 | ||||||||
Appendix B
| Family | Taxon | Origin | Source |
|---|---|---|---|
| Elapidae | Bungarus multicinctus | China | SVRI |
| Dendroaspis polylepis | Kenya | Biotoxins | |
| Micrurus surinamensis | Letícia, Colombia | CEPB | |
| Naja kaouthia | Unknown | KRZ | |
| Naja sputatrix | Java | Zooherp | |
| Ophiophagus hannah | Unknown | KRZ | |
| Viperidae | Bitis gabonica | Burundi | Zooherp |
| Bitis nasicornis | Unknown | Zooherp | |
| Cerastes cerastes | Unknown | KRZ | |
| Daboia palestinae | Unknown | KRZ | |
| Daboia russellii siamensis | Thailand | SVRI | |
| Pseudocerastes fieldi | Unknown | KRZ | |
| Crotalidae | Agkistrodon contortrix contortrix | South Carolina | Biotoxins |
| Agkistrodon piscivorus leucostoma | Missouri | KRZ | |
| Atropoides nummifer | SE Mexico | KRZ | |
| Bothriechis schlegelii | Costa Rica | Clodomiro Picado | |
| Bothrops moojeni | Goiás, Brasil | CEPB | |
| Bothrops erythromelas | NE Brazil | KRZ | |
| Calloselasma rhodostoma | Thailand | KRZ | |
| Cerrophidion sasai | Costa Rica | Clodomiro Picado | |
| Crotalus adamanteus | Florida | Biotoxins | |
| Crotalus durissus terrificus | Brazil | CEPB | |
| Crotalus cerastes | Yuma, Arizona | SDA | |
| Crotalus mitchellii pyrrhus | Yavapai, Arizona | SDA | |
| Crotalus viridis concolor | Rio Blanco, Colorado | SDA | |
| Crotalus viridis viridis | Rio Blanco, Colorado | SDA | |
| Lachesis stenophrys | Costa Rica | Clodomiro Picado | |
| Ovophis okinavensis | Okinawa | SDA | |
| Protobothrops elegans | Itoman, Okinawa | SDA | |
| Protobothrops flavoviridis | Itoman, Okinawa | SDA | |
| Protobothrops mucrosquamatus | Nago, Okinawa | SDA |
References
- Sasaki, T. Chemical studies on the venom of Formosan habu (Trimeresurus mucrosquamatus Cantor). III. On the dialyzable substances in the venom. J. Pharmacol. Soc. Jpn. 1960, 80, 844. [Google Scholar]
- Shipolini, R.; Ivanov, C.P.; Dimitrov, G.; Alexiev, B.V. Composition of the low molecular fraction of the Bulgarian viper venom. Biochim. Biophys. Acta (BBA) Gen. Subj. 1965, 104, 292–295. [Google Scholar] [CrossRef]
- Merkel, P.; Beck, A.; Muhammad, K.; Ali, S.A.; Schönfeld, C.; Voelter, W.; Duszenko, M. Spermine isolated and identified as the major trypanocidal compound from the snake venom of Eristocophis macmahoni causes autophagy in Trypanosoma brucei. Toxicon 2007, 50, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Grishin, E.V.; Volkova, T.M.; Arsen’ev, A.S.; Reshetova, O.S.; Onoprienko, V.V. Structural-functional characteristics of argiopine—The ion channel blockers from the spider Argiope lobata venom. Bioorg. Khim 1986, 12, 1121–1124. [Google Scholar] [PubMed]
- Adams, M.E.; Carney, R.L.; Enderlin, F.E.; Fu, E.T.; Jarema, M.A.; Li, J.P.; Miller, C.A.; Schooley, D.A.; Shapiro, M.J.; Venema, V.J. Structures and biological activities of three synaptic antagonists from orb weaver spider venom. Biochem. Biophys. Res. Commun. 1987, 148, 678–683. [Google Scholar] [CrossRef]
- Budd, T.; Clinton, P.; Dell, A.; Duce, I.R.; Johnson, S.J.; Quicke, D.L.; Taylor, G.W.; Usherwood, P.N.; Usoh, G. Isolation and characterisation of glutamate receptor antagonists from venoms of orb-web spiders. Brain Res. 1988, 448, 30–39. [Google Scholar] [CrossRef]
- Adams, M.E.; Herold, E.E.; Venema, V.J. Two classes of channel-specific toxins from funnel web spider venom. J. Comp. Physiol. A 1989, 164, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Pan-Hou, H.; Hidemitsu, P.-H.; Yasuo, S.; Masao, S.; Masanori, Y.; Nobufumi, K. A spider toxin (JSTX) inhibits l-glutamate uptake by rat brain synaptosomes. Brain Res. 1989, 476, 354–357. [Google Scholar] [CrossRef]
- Ippolito, J.E.; Xu, J.; Jain, S.; Moulder, K.; Mennerick, S.; Crowley, J.R.; Townsend, R.R.; Gordon, J.I. An integrated functional genomics and metabolomics approach for defining poor prognosis in human neuroendocrine cancers. Proc. Natl. Acad. Sci. USA 2005, 102, 9901–9906. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, M.R.; Keinänen, T.A.; Jouko, V.; Khomutov, A.R.; Leena, A.; Juhani, J.; Seppo, A. Analysis of underivatized polyamines by reversed phase liquid chromatography with electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ran, L.; Kaishun, B.; Ying, J.; Qian, W.; Ran, Y.; Qing, L. Determination of polyamines in human plasma by high-performance liquid chromatography coupled with Q-TOF mass spectrometry. J. Mass Spectrom. 2012, 47, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Aird, S.D.; Aggarwal, S.; Villar-Briones, A.; Tin, M.M.-Y.; Terada, K.; Mikheyev, A.S. Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly. BMC Genom. 2015, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K. Factors associated with the mass of venom expended by prairie rattlesnakes (Crotalus v. viridis) feeding on mice. Toxicon 1992, 30, 449–460. [Google Scholar] [CrossRef]
- Morrison, J.J.; Pearn, J.H.; Coulter, A.R. The mass of venom injected by two elapidae: The taipan (Oxyuranus scutellatus) and the Australian tiger snake (Notechis scutatus). Toxicon 1982, 20, 739–745. [Google Scholar] [CrossRef]
- Morrison, J.J.; Pearn, J.H.; Charles, N.T.; Coulter, A.R. Further studies on the mass of venom injected by Elapid snakes. Toxicon 1983, 21, 279–284. [Google Scholar] [CrossRef]
- Cooper, K.D.; Shukla, J.B.; Rennert, O.M. Polyamine distribution in cellular compartments of blood and in aging erythrocytes. Clin. Chim. Acta 1976, 73, 71–88. [Google Scholar] [CrossRef]
- Uehara, N.; Shirakawa, S.; Uchino, H.; Saeki, Y. Elevated contents of spermidine and spermine in the erythrocytes of cancer patients. Cancer 1980, 45, 108–111. [Google Scholar] [CrossRef]
- Tabor, C.W.; Rosenthal, S.M. Pharmacology of spermine and spermidine; some effects on animals and bacteria. J. Pharmacol. Exp. Ther. 1956, 116, 139–155. [Google Scholar] [PubMed]
- Tabor, H.; Tabor, C.W.; Rosenthal, S.M. The Biochemistry of the Polyamines: Spermidine and Spermine. Annu. Rev. Biochem. 1961, 30, 579–604. [Google Scholar] [CrossRef]
- Bachrach, U. Oxidized polyamines. Ann. N. Y. Acad. Sci. 1970, 171, 939–956. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; Watanabe, Y.; Villar-Briones, A.; Roy, M.C.; Terada, K.; Mikheyev, A.S. Quantitative high-throughput profiling of snake venom gland transcriptomes and proteomes (Ovophis okinavensis and Protobothrops flavoviridis). BMC Genom. 2013, 14, 790. [Google Scholar] [CrossRef] [PubMed]
- DiScenna, P.G.; Ferchmin, P.A.; Eterovic, V.A.; Teyler, T.J. Spermine depresses NMDA, K/AMPA and GABAA-mediated synaptic transmission in the rat hippocampal slice preparation. Brain Res. 1994, 647, 353–356. [Google Scholar] [CrossRef]
- Rosenthal, S.M.; Tabor, C.W. The pharmacology of spermine and spermidine; distribution and excretion. J. Pharmacol. Exp. Ther. 1956, 116, 131–138. [Google Scholar] [PubMed]
- Michaelson, I.A.; Coffman, P.Z. Histamine and spermidine in tissues of the guinea pig. Biochem. Pharmacol. 1967, 16, 1636–1641. [Google Scholar] [CrossRef]
- Shimizu, H.; Kakimoto, Y.; Sano, I. The determination and distribution of polyamines in mammalian nervous system. J. Pharmacol. Exp. Ther. 1964, 143, 199–204. [Google Scholar] [PubMed]
- Perry, T.L.; Hansen, S.; Foulks, J.G.; Ling, G.M. Aliphatic and aromatic amines of cat brain. J. Neurochem. 1965, 12, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Paschen, W. Polyamine metabolism in different pathological states of the brain. Mol. Chem. Neuropathol. 1992, 16, 241–271. [Google Scholar] [CrossRef] [PubMed]
- Zappia, V.; Porta, R.; Cartenì-Farina, M.; De Rosa, M.; Gambacorta, A. Polyamine distribution in eukaryotes: Occurrence of sym-nor-spermidine and sym-nor-spermine in arthropods. FEBS Lett. 1978, 94, 161–165. [Google Scholar] [CrossRef]
- Polticelli, F.; Salvi, D.; Mariottini, P.; Amendola, R.; Cervelli, M. Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evol. Biol. 2012, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Chaisiri, P.; Harper, M.E.; Griffiths, K. Plasma spermine concentrations of patients with benign and malignant tumours of the breast or prostate. Clin. Chim. Acta 1979, 92, 273–282. [Google Scholar] [CrossRef]
- Masuko, T.; Kusama-Eguchi, K.; Sakata, K.; Kusama, T.; Chaki, S.; Okuyama, S.; Williams, K.; Kashiwagi, K.; Igarashi, K. Polyamine transport, accumulation, and release in brain. J. Neurochem. 2003, 84, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Cino, I.; Formenti, A. Spermine biphasically affects N-type calcium channel currents in adult dorsal root ganglion neurons of the rat. Biochim. Biophys. Acta 2008, 1778, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Aird, S.D. Taxonomic distribution and quantitative analysis of free purine and pyrimidine nucleosides in snake venoms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Giampietro, D.E. An activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003, 26, 9–11. [Google Scholar] [CrossRef]
- Yoshida, T.; Nagai, R.; Hashimoto, T. Lack of species specificity of a skin-reactive factor released from sensitized guinea pig spleen cells. Lab. Investig. 1973, 29, 329–335. [Google Scholar] [PubMed]
- Hashimoto, H.; Unemoto, T.; Hayashi, M. Inhibitory action of spermine on the contractions of rat uterus. Am. J. Physiol. 1973, 225, 743–746. [Google Scholar] [PubMed]
- Nicchitta, C.V.; Williamson, J.R. Spermine. A regulator of mitochondrial calcium cycling. J. Biol. Chem. 1984, 259, 12978–12983. [Google Scholar] [PubMed]
- De Meis, L. Relaxing effect of spermine and spermidine on intact and glycerol-treated muscle. Am. J. Physiol. 1967, 212, 92–96. [Google Scholar] [PubMed]
- Marmo, E.; Berrino, L.; Cazzola, M.; Filippelli, A.; Cafaggi, G.; Persico, N.; Spadaro, R.; Nisticò, G. Cardiovascular and respiratory effects of spermidine and spermine: An experimental study. Biomed. Biochim. Acta 1984, 43, 509–515. [Google Scholar] [PubMed]
- Chideckel, E.W.; Fedan, J.S.; Mike, P. Polyamines and putreanine relax respiratory tract smooth muscle in the guinea-pig. Eur. J. Pharmacol. 1985, 116, 187–190. [Google Scholar] [CrossRef]
- Chideckel, E.W.; Dedhia, H.H.; Fedan, J.S.; Teba, L.; Jain, A. Spermine decreases peripheral vascular resistance in the dog and relaxes the isolated aorta of the guinea pig. Cardiovasc. Res. 1986, 20, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.I.; Cantabrana, B.; Sánchez, M.; Hidalgo, A. Extracellular and intracellular effects of polyamines on smooth muscle contractions. Life Sci. 1995, 57, 855–861. [Google Scholar] [CrossRef]
- Nilsson, B.O.; Gomez, M.; Santiago Carrilho, R.; Nordström, I.; Hellstrand, P. Differential actions of exogenous and intracellular spermine on contractile activity in smooth muscle of rat portal vein. Acta Physiol. Scand. 1995, 154, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.C.; Oh, S.Y.; Kim, K.D.; Kim, S.C.; Lee, M.Y. Effects of spermine on the relaxation response of rat detrusor smooth muscles. Eur. J. Pharmacol. 2007, 573, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.P.; Cantabrana, B.; Sánchez, M.; Hidalgo, A. Effect of spermine and alpha-difluoromethylornithine on KCl- and CaCl2-induced contraction in rat uterine smooth muscle. J. Auton. Pharmacol. 1998, 18, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Hellstrand, P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995, 430, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.H.; Absi, M.; Ward, D.T.; Ohanian, J.; Dodd, R.H.; Dauban, P.; Petrel, C.; Ruat, M.; Edwards, G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and Calhex 231. Circ. Res. 2005, 97, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.J.; Ye, C.P.; Diaz, R.; Kifor, O.; Bai, M.; Vassilev, P.; Brown, E. The Ca2+-sensing receptor: A target for polyamines. Am. J. Physiol. 1997, 273, C1315–C1323. [Google Scholar] [PubMed]
- Sham, J.S.; Cleemann, L.; Morad, M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ziegelstein, R.C.; Xiong, Y.; He, C.; Hu, Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006, 342, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Ferroni, C.; Flamigni, F.; Stefanelli, C.; Capogrossi, M.C. Polyamine effects on [Ca2+]i homeostasis and contractility in isolated rat ventricular cardiomyocytes. Am. J. Physiol. 1994, 267, H587–H592. [Google Scholar] [PubMed]
- Guevara-Balcázar, G.; Querejeta-Villagómez, E.; Nuevo-Adalla, O.; Orozco-Guillen, A.; Rubio-Gayosso, I.; Hernández-Castillo, J.R.; Zamora-Garza, M.; Ceballos-Reyes, G. Spermine-induced negative inotropic effect in isolated rat heart, is mediated through the release of ATP. Biochem. Pharmacol. 2003, 66, 157–161. [Google Scholar] [CrossRef]
- Reid, C.A.; Bekkers, J.M.; Clements, J.D. Presynaptic Ca2+ channels: A functional patchwork. Trends Neurosci. 2003, 26, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Bonci, A.; Grillner, P.; Mercuri, N.B.; Bernardi, G. L-Type calcium channels mediate a slow excitatory synaptic transmission in rat midbrain dopaminergic neurons. J. Neurosci. 1998, 18, 6693–6703. [Google Scholar] [PubMed]
- Herman, M.D.; Reuveny, E.; Narahashi, T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J. Physiol. 1993, 462, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Eterović, V.A.; Torres, E.; Ferchmin, P.A. Spermine does not compete with omega-conotoxin GVIA in the striatum radiatum of the hippocampal slice. Brain Res. 1997, 772, 191–202. [Google Scholar] [CrossRef]
- Pullan, L.M.; Keith, R.A.; LaMonte, D.; Stumpo, R.J.; Salama, A.I. The polyamine spermine affects omega-conotoxin binding and function at N-type voltage-sensitive calcium channels. J. Auton. Pharmacol. 1990, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, H. Polyamines allosterically modulate [3H]nitrendipine binding to the voltage-sensitive calcium channel in rat brain. Eur. J. Pharmacol. 1992, 225, 167–169. [Google Scholar] [CrossRef]
- Joshi, D.C.; Singh, M.; Krishnamurthy, K.; Joshi, P.G.; Joshi, N.B. AMPA induced Ca2+ influx in motor neurons occurs through voltage gated Ca2+ channel and Ca2+ permeable AMPA receptor. Neurochem. Int. 2011, 59, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Cayzac, S.H.; Rocher, A.; Obeso, A.; Gonzalez, C.; Riccardi, D.; Kemp, P.J. Spermine attenuates carotid body glomus cell oxygen sensing by inhibiting L-type Ca2(+) channels. Respir. Physiol. Neurobiol. 2011, 175, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.; Ronca-Testoni, S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: Modulation by endogenous effectors, drugs and disease states. Pharmacol. Rev. 1997, 49, 1–51. [Google Scholar] [PubMed]
- Uehara, A.; Fill, M.; Vélez, P.; Yasukochi, M.; Imanaga, I. Rectification of rabbit cardiac ryanodine receptor current by endogenous polyamines. Biophys. J. 1996, 71, 769–777. [Google Scholar] [CrossRef]
- Zarka, A.; Shoshan-Barmatz, V. The interaction of spermine with the ryanodine receptor from skeletal muscle. Biochim. Biophys. Acta 1992, 1108, 13–20. [Google Scholar] [CrossRef]
- MacDermott, A.B.; Mayer, M.L.; Westbrook, G.L.; Smith, S.J.; Barker, J.L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 1986, 321, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Brackley, P.; Goodnow, R., Jr.; Nakanishi, K.; Sudan, H.L.; Usherwood, P.N. Spermine and philanthotoxin potentiate excitatory amino acid responses of Xenopus oocytes injected with rat and chick brain RNA. Neurosci. Lett. 1990, 114, 51–56. [Google Scholar] [CrossRef]
- McGurk, J.F.; Bennett, M.V.; Zukin, R.S. Polyamines potentiate responses of N-methyl-d-aspartate receptors expressed in xenopus oocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 9971–9974. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Miyazaki, M.; Mizuno, S.; Takigawa, M.; Hirose, T.; Nishimura, K.; Toida, T.; Williams, K.; Kashiwagi, K.; Igarashi, K. The pore region of N-methyl-d-aspartate receptors differentially influences stimulation and block by spermine. J. Pharmacol. Exp. Ther. 2008, 327, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Excitotoxic cell death. J. Neurobiol. 1992, 23, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Zito, K.; Scheuss, V. NMDA Receptor Function and Physiological Modulation. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 1157–1164. [Google Scholar]
- Chitravanshi, V.C.; Sapru, H.N. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996, 715, 104–112. [Google Scholar] [CrossRef]
- Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J. Neurosci. 2008, 28, 6826–6835. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.J. Development of synaptic transmission to respiratory motoneurons. Respir. Physiol. Neurobiol. 2011, 179, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Deliagina, T.; Ekeberg, O.; el Manira, A.; Hill, R.H.; Lansner, A.; Orlovsky, G.N.; Wallén, P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995, 18, 270–279. [Google Scholar] [CrossRef]
- Grillner, S.; Wallén, P.; Hill, R.; Cangiano, L.; El Manira, A. Ion channels of importance for the locomotor pattern generation in the lamprey brainstem-spinal cord. J. Physiol. 2001, 533, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Romano, C.; Dichter, M.A.; Molinoff, P.B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991, 48, 469–498. [Google Scholar] [CrossRef]
- Marvizón, J.-C.; Juan-Carlos, M.; Michel, B. NMDA receptor activation by spermine requires glutamate but not glycine. Eur. J. Pharmacol. Mol. Pharmacol. 1993, 244, 103–104. [Google Scholar] [CrossRef]
- Pullan, L.M.; Powel, R.J. Spermine reciprocally changes the affinity of NMDA receptor agonists and antagonists. Eur. J. Pharmacol. 1991, 207, 173–174. [Google Scholar] [CrossRef]
- Benveniste, M.; Mayer, M.L. Multiple effects of spermine on N-methyl-d-aspartic acid receptor responses of rat cultured hippocampal neurones. J. Physiol. 1993, 464, 131–163. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, L.J.; Boxer, P.A. Polyamine modulation of excitatory amino acid responses in the rat cortical wedge. Neuropharmacology 1993, 32, 1025–1035. [Google Scholar] [CrossRef]
- Araneda, R.C.; Zukin, R.S.; Bennett, M.V. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: A whole-cell and single-channel study. Neurosci. Lett. 1993, 152, 107–112. [Google Scholar] [CrossRef]
- Ferchmin, P.A.; Pérez, D.; Biello, M. Spermine is neuroprotective against anoxia and N-methyl-d-aspartate in hippocampal slices. Brain Res. 2000, 859, 273–279. [Google Scholar] [CrossRef]
- Segal, J.A.; Skolnick, P. Spermine-induced toxicity in cerebellar granule neurons is independent of its actions at NMDA receptors. J. Neurochem. 2000, 74, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ran, I.; Israeli, R.; Miura, R.M.; Ernest, P. Spermine modulates neuronal excitability and NMDA receptors in juvenile gerbil auditory thalamus. Hear. Res. 2003, 176, 65–79. [Google Scholar] [CrossRef]
- Turecek, R.; Vlcek, K.; Petrovic, M.; Horak, M.; Vlachova, V.; Vyklicky, L., Jr. Intracellular spermine decreases open probability of N-methyl-d-aspartate receptor channels. Neuroscience 2004, 125, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Calderón, F.; López-Colomé, A.M. Spermine inhibits [3H]glycine binding at the NMDA receptors from plexiform layers of chick retina. Neurochem. Res. 1998, 23, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.; Matsumoto, I.; Niwa, S.; Dodd, P.R. The modulatory effect of spermine on the glutamate-NMDA receptor is regionally variable in normal human adult cerebral cortex. Pharmacol. Toxicol. 1999, 84, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, L.; Mortensen, M.; Dodd, P.R.; Lewis, R.J. Spermine modulation of the glutamate(NMDA) receptor is differentially responsive to conantokins in normal and Alzheimer’s disease human cerebral cortex. J. Neurochem. 2002, 81, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.M.; Shaw, G.G. Investigation of the involvement of the N-methyl-d-aspartate receptor macrocomplex in the development of spermine-induced CNS excitation in vivo. Br. J. Pharmacol. 1996, 117, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.P.; Shaw, G.G. Effect of spermine and N1-dansyl-spermine on epileptiform activity in mouse cortical slices. Eur. J. Pharmacol. 2005, 524, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Adriani, W.; Felici, A.; Sargolini, F.; Roullet, P.; Usiello, A.; Oliverio, A.; Mele, A. N-methyl-d-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp. Brain Res. 1998, 123, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Barraco, R.A.; Coffin, V.L.; Altman, H.J.; Phillis, J.W. Central effects of adenosine analogs on locomotor activity in mice and antagonism of caffeine. Brain Res. 1983, 272, 392–395. [Google Scholar] [CrossRef]
- Karlsten, R.; Rolf, K.; Torsten, G.; Per, H.; Claes, P. Effects of Intrathecal Injection of the Adenosine Receptor Agonists R-Phenylisopropyl-Adenosine and N-Ethylcarboxamide-Adenosine on Nociception and Motor Function in the Rat. Anesth. Analg. 1990, 71, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Nikodijević, O.; Sarges, R.; Daly, J.W.; Jacobson, K.A. Behavioral effects of A1- and A2-selective adenosine agonists and antagonists: Evidence for synergism and antagonism. J. Pharmacol. Exp. Ther. 1991, 259, 286–294. [Google Scholar] [PubMed]
- Jain, N.; Kemp, N.; Adeyemo, O.; Buchanan, P.; Stone, T.W. Anxiolytic activity of adenosine receptor activation in mice. Br. J. Pharmacol. 1995, 116, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987, 7, 369–379. [Google Scholar] [PubMed]
- Tymianski, M.; Charlton, M.P.; Carlen, P.L.; Tator, C.H. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 1993, 13, 2085–2104. [Google Scholar] [PubMed]
- Rao, T.S.; Cler, J.A.; Oei, E.J.; Emmett, M.R.; Mick, S.J.; Iyengar, S.; Wood, P.L. The polyamines, spermine and spermidine, negatively modulate N-methyl-d-aspartate (NMDA) and quisqualate receptor mediated responses in vivo: Cerebellar cyclic GMP measurements. Neurochem. Int. 1990, 16, 199–206. [Google Scholar] [CrossRef]
- Brundell, P.; Goodnow, R., Jr.; Kerry, C.J.; Nakanishi, K.; Sudan, H.L.; Usherwood, P.N. Quisqualate-sensitive glutamate receptors of the locust Schistocerca gregaria are antagonised by intracellularly applied philanthotoxin and spermine. Neurosci. Lett. 1991, 131, 196–200. [Google Scholar] [CrossRef]
- Otsuki, M.; Davidson, M.; Goodenough, S.; Wilce, P.A.; Tase, C.; Matsumoto, I. In vivo pharmacological study of spermine-induced neurotoxicity. Neurosci. Lett. 1995, 196, 81–84. [Google Scholar] [CrossRef]
- Isa, T.; Iino, M.; Ozawa, S. Spermine blocks synaptic transmission mediated by Ca(2+)-permeable AMPA receptors. Neuroreport 1996, 7, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Washburn, M.S.; Dingledine, R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J. Pharmacol. Exp. Ther. 1996, 278, 669–678. [Google Scholar] [PubMed]
- Noh, K.-M.; Yokota, H.; Mashiko, T.; Castillo, P.E.; Zukin, R.S.; Bennett, M.V.L. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA 2005, 102, 12230–12235. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, L.; Vandenberghe, W.; Klaassen, H.; Van Houtte, E.; Robberecht, W. Ca(2+)-permeable AMPA receptors and selective vulnerability of motor neurons. J. Neurol. Sci. 2000, 180, 29–34. [Google Scholar] [CrossRef]
- Van Damme, P.; Van Den Bosch, L.; Van Houtte, E.; Callewaert, G.; Robberecht, W. GluR2-dependent properties of AMPA receptors determine the selective vulnerability of motor neurons to excitotoxicity. J. Neurophysiol. 2002, 88, 1279–1287. [Google Scholar] [PubMed]
- Corona, J.C.; Tapia, R. Ca2+-permeable AMPA receptors and intracellular Ca2+ determine motoneuron vulnerability in rat spinal cord in vivo. Neuropharmacology 2007, 52, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Perrais, D.; Veran, J.; Mulle, C. Gating and permeation of kainate receptors: Differences unveiled. Trends Pharmacol. Sci. 2010, 31, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Anis, N.; Sherby, S.; Goodnow, R., Jr.; Niwa, M.; Konno, K.; Kallimopoulos, T.; Bukownik, R.; Nakanishi, K.; Usherwood, P.; Eldefrawi, A. Structure-activity relationships of philanthotoxin analogs and polyamines on N-methyl-d-aspartate and nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 1990, 254, 764–773. [Google Scholar] [PubMed]
- Szczawinska, K.; Ferchmin, P.A.; Hann, R.M.; Eterović, V.A. Electric organ polyamines and their effects on the acetylcholine receptor. Cell. Mol. Neurobiol. 1992, 12, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.S. Modulation of the nicotinic acetylcholine receptor channels by spermine in Xenopus muscle cell culture. Neurosci. Lett. 1994, 182, 99–103. [Google Scholar] [CrossRef]
- Haghighi, A.P.; Cooper, E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J. Neurosci. 1998, 18, 4050–4062. [Google Scholar] [PubMed]
- Law, C.L.; Wong, P.C.; Fong, W.F. Effects of polyamines on the uptake of neurotransmitters by rat brain synaptosomes. J. Neurochem. 1984, 42, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Tsvilovskyy, V.V.; Zholos, A.V.; Bolton, T.B. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br. J. Pharmacol. 2004, 143, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, R.M.; De Luca, G.; Nisticò, G.; Ientile, R. gamma-Aminobutyric acid metabolism and behavioral effects after intraventricular injection of spermine in chicks. J. Neurochem. 1985, 45, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Gilad, G.M.; Gilad, V.H.; Wyatt, R.J. Polyamines modulate the binding of GABAA-benzodiazepine receptor ligands in membranes from the rat forebrain. Neuropharmacology 1992, 31, 895–898. [Google Scholar] [CrossRef]
- Sábato, U.C.; Aguilar, J.S.; De Robertis, E. Benzodiazepine receptors in rat brain: Action of Triton X-100 and localization in relation to the synaptic region. J. Recept. Res. 1981, 2, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Martijena, I.D.; Salvatierra, N.A.; Arce, A. Benzodiazepine receptor recruitment after acute stress in synaptosomal membranes from forebrain of young chicks: Action of Triton X-100. J. Neural Transm. Gen. Sect. 1992, 87, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yermolaieva, O.; Leonard, A.S.; Schnizler, M.K.; Abboud, F.M.; Welsh, M.J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA. 2004, 101, 6752–6757. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, Y.-Z.; Yang, T.; Chu, X.-P.; Yu, Y.; Huang, Y.; Cao, H.; Hansen, J.; Simon, R.P.; Zhu, M.X.; et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J. Neurosci. 2011, 31, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.P.; Wang, X.; Miyares, R.L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J. Biol. Chem. 2006, 281, 8991–8995. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, C.; da Silva, M.A.; dos Santos, G.T.; Rossato, M.F.; de Oliveira, S.M.; Drewes, C.C.; Pazini, A.M.; Guerra, G.P.; Rubin, M.A.; Ferreira, J. Contribution of peripheral vanilloid receptor to the nociception induced by injection of spermine in mice. Pharmacol. Biochem. Behav. 2011, 99, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Israels, S.J.; Gerrard, J.M.; Robinson, P. Differential effects of spermine on aggregation, inositol phosphate formation and protein phosphorylation in human platelets in response to thrombin, arachidonic acid and lysophosphatidic acid. Biochim. Biophys. Acta 1986, 883, 247–252. [Google Scholar] [CrossRef]
- Joseph, S.; Krishnamurthi, S.; Kakkar, V.V. Effect of the polyamine-spermine on agonist-induced human platelet activation--specific inhibition of “aggregation-independent” events induced by thrombin, but not by collagen, thromboxane mimetic, phorbol ester or calcium ionophore. Thromb. Haemost. 1987, 57, 191–195. [Google Scholar] [PubMed]
- Via, L.D.; Mariangela, F.; Mario, M.; Paola, P.; De Marco, L.; Vecchia, F.D.; Nicoletta, R.; Renzo, D. On the Mechanism of the Spermine-Exerted Inhibition on α-Thrombin-Induced Platelet Activation*. Thromb. Res. 2000, 98, 59–71. [Google Scholar] [CrossRef]
- De la Peña, N.C.; Sosa-Melgarejo, J.A.; Ramos, R.R.; Méndez, J.D. Inhibition of platelet aggregation by putrescine, spermidine, and spermine in hypercholesterolemic rabbits. Arch. Med. Res. 2000, 31, 546–550. [Google Scholar] [CrossRef]
- Hughes, G.; Starling, A.P.; East, J.M.; Lee, A.G. Mechanism of inhibition of the Ca(2+)-ATPase by spermine and other polycationic compounds. Biochemistry 1994, 33, 4745–4754. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Sepúlveda, M.R.; Salvador, J.M.; Mata, A.M. Effect of spermine on the activity of synaptosomal plasma membrane Ca(2+)-ATPase reconstituted in neutral or acidic phospholipids. Biochim. Biophys. Acta 2003, 1611, 197–203. [Google Scholar] [CrossRef]
- Carafoli, E. The calcium pumping ATPase of the plasma membrane. Annu. Rev. Physiol. 1991, 53, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, Y.V.; Lang, F. Inhibition of cation channels in human erythrocytes by spermine. J. Membr. Biol. 2010, 237, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Trout, J.J.; Koenig, H.; Goldstone, A.D.; Lu, C.Y. Blood-brain barrier breakdown by cold injury. Polyamine signals mediate acute stimulation of endocytosis, vesicular transport, and microvillus formation in rat cerebral capillaries. Lab. Investig. 1986, 55, 622–631. [Google Scholar] [PubMed]
- Glantz, L.; Nates, J.L.; Trembovler, V.; Bass, R.; Shohami, E. Polyamines induce blood-brain barrier disruption and edema formation in the rat. J. Basic Clin. Physiol. Pharmacol. 1996, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koenig, H.; Harold, K.; Goldstone, A.D.; Lu, C.Y. Polyamines mediate the reversible opening of the blood-brain barrier by the intracarotid infusion of hyperosmolal mannitol. Brain Res. 1989, 483, 110–116. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Curran, G.L. Polyamine modification increases the permeability of proteins at the blood-nerve and blood-brain barriers. J. Neurochem. 1996, 66, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L. Increased permeability of superoxide dismutase at the blood-nerve and blood-brain barriers with retained enzymatic activity after covalent modification with the naturally occurring polyamine, putrescine. J. Neurochem. 1996, 67, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Paik Jung, H.Y.; Bjeldanes, L.F. Effects of cadaverine on histamine transport and metabolism in isolated gut sections of the guinea-pig. Food Cosmet. Toxicol. 1979, 17, 629–632. [Google Scholar] [CrossRef]
- Mongar, J.L. Effect of chain length of aliphatic amines on histamine potentiation and release. Br. J. Pharmacol. Chemother. 1957, 12, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.E.; Beery, J.T.; Lyons, S.A.; Taylor, S.L. Cadaverine and aminoguanidine potentiate the uptake of histamine in vitro in perfused intestinal segments of rats. Toxicol. Appl. Pharmacol. 1983, 70, 445–458. [Google Scholar] [CrossRef]
- Taylor, S.L.; Lieber, E.R. In vitro inhibition of rat intestinal histamine-metabolizing enzymes. Food Cosmet. Toxicol. 1979, 17, 237–240. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [PubMed]
- Hui, J.Y.; Taylor, S.L. Inhibition of in vivo histamine metabolism in rats by foodborne and pharmacologic inhibitors of diamine oxidase, histamine N-methyltransferase, and monoamine oxidase. Toxicol. Appl. Pharmacol. 1985, 81, 241–249. [Google Scholar] [CrossRef]
- Rossi, F.; Nisticò, G.; De Marco, G.; Berrino, L.; Matera, C.; Bile, G.; Marmo, E. Cardiovascular effects of putrescine in dogs after systemic, intra-arterial vertebral and intraventricular injection. Res. Commun. Chem. Pathol. Pharmacol. 1984, 46, 43–52. [Google Scholar] [PubMed]
- Bowsher, R.R.; Verburg, K.M.; Henry, D.P. Rat histamine N-methyltransferase. Quantification, tissue distribution, purification, and immunologic properties. J. Biol. Chem. 1983, 258, 12215–12220. [Google Scholar] [PubMed]
- Schwelberger, H.G.; Hittmair, A.; Kohlwein, S.D. Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy. Inflamm. Res. 1998, 47, S60–S61. [Google Scholar] [CrossRef] [PubMed]
- De Meis, L.; De Paula, H.J. Polyamines and muscle relaxation. Inhibition of actomyosin ATPase activity by spermine and spermidine. Arch. Biochem. Biophys. 1967, 119, 16–21. [Google Scholar] [CrossRef]
- Shou-hsian Mao Food of the Common Venomous Snakes of Taiwan. Herpetologica 1970, 26, 45–48.
- Branch, W.R.; Haagner, G.V.; Shine, R. Is there an ontogenetic shift in mamba diet? Taxonomic confusion and dietary records for black and green mambas (Dendroaspis: Elapidae). Herpetol. Nat. Hist 1995. [Google Scholar]
- Olamendi-Portugal, T.; Batista, C.V.; Restano-Cassulini, R.; Pando, V.; Villa-Hernandez, O.; Zavaleta-Martinez-Vargas, A.; Salas-Arruz, M.C.; Rodriguez de la Vega, R.C.; Becerril, B.; Possani, L.D. Proteomic analysis of the venom from the fish eating coral snake Micrurus surinamensis: Novel toxins, their function and phylogeny. Proteomics 2008, 8, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.H.; Ávila, R.W.; Kawashita-Ribeiro, R.A. Squamata, Elapidae, Micrurus surinamensis (Cuvier, 1817): New records and distribution map in the state of Mato Grosso, Brazil, with notes on diet and activity period. Check List 2011, 7, 350–351. [Google Scholar]
- Chanhome, L.; Lawan, C.; Piboon, J.; Henry, W.; Cox, M.J. Venomous snake husbandry in Thailand. Wilderness Environ. Med. 2001, 12, 17–23. [Google Scholar] [CrossRef]
- Modahl, C.M.; Mukherjee, A.K.; Mackessy, S.P. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia). Toxicon 2016, 119, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Luiselli, L.; Luca, L.; Akani, G.C. Diet of sympatric Gaboon Vipers (Bitis gabonica) and Nose-horned Vipers (Bitis nasicornis) in southern Nigeria. Afr. J. Herpetol. 2003, 52, 101–106. [Google Scholar] [CrossRef]
- Bazaa, A.; Marrakchi, N.; El Ayeb, M.; Sanz, L.; Calvete, J.J. Snake venomics: Comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics 2005, 5, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, J.F.; Hall, H.P.; Casey, E.R.; Hayes, W.K. Neurotropic effects of venoms and other factors that promote prey acquisition. J. Exp. Zool. A Ecol. Genet. Physiol. 2007, 307, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Jackson, T.N.W.; Casewell, N.R.; Low, D.H.W.; Rossi, S.; Baumann, K.; Fathinia, B.; Visser, J.; Nouwens, A.; Hendrikx, I.; et al. Extreme venom variation in Middle Eastern vipers: A proteomics comparison of Eristicophis macmahonii, Pseudocerastes fieldi and Pseudocerastes persicus. J. Proteomics 2015, 116, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Trauth, S.E. Vertebrate Prey of Selected Arkansas Snakes. Proc. Ark. Acad. Sci. 1995, 46, 188–192. [Google Scholar]
- Heatwole, H.; Harold, H.; Naomie, P.; Peter, K. Ontogenetic Changes in the Resistance of Bullfrogs (Rana catesbeiana) to the Venom of Copperheads (Agkistrodon contortrix contortrix) and Cottonmouths (Agkistrodon piscivorus piscivorus). Copeia 1999, 1999, 808–814. [Google Scholar] [CrossRef]
- Himes, J.; John, H. Diet composition of Nerodia sipedon (Serpentes: Colubridae) and its dietary overlap with, and chemical recognition of Agkistrodon piscivorus (Serpentes: Viperidae). Amphib-reptil. 2003, 24, 181–188. [Google Scholar] [CrossRef]
- Vincent, S.E.; Anthony, H.; Irschick, D.J. Ontogeny of intersexual head shape and prey selection in the pitviper Agkistrodon piscivorus. Biol. J. Linn. Soc. Lond. 2004, 81, 151–159. [Google Scholar] [CrossRef]
- Martins, M.; Marques, O.A.V.; Sazima, I. Ecological and phylogenetic correlates of feeding habits in Neotropical pitvipers of the genus Bothrops. In Biology of the Vipers; Schuett, G.W., Hoggren, M., Douglas, M.E., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2002; pp. 1–22. [Google Scholar]
- Greene, H.W. Species richness in tropical predators. In Tropical Rainforests: Diversity and Conservation; Almeda, F., Pringle, C.M., Eds.; Memoirs California Academy of Science: San Francisco, CA, USA, 1988; pp. 259–279. [Google Scholar]
- Sorrell, G. Natural History and Conservation of the Eyelash Palmpitviper (Bothriechis schlegelii) in Western Panamá. Master’s Theise, Auburn University, Auburn, AL, USA, 2007. [Google Scholar]
- Nogueira, C.; Cristiano, N.; Sawaya, R.J.; Marcio, M. Ecology of the Pitviper, Bothrops moojeni, in the Brazilian Cerrado. J. Herpetol. 2003, 37, 653–659. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wolfgang, W.; Thorpe, R.S. Intraspecific Variation in the Feeding Ecology of the Crotaline Snake Calloselasma rhodostoma in Southeast Asia. J. Herpetol. 1998, 32, 198–205. [Google Scholar] [CrossRef]
- Luiselli, L. Broad geographic, taxonomic and ecological patterns of interpopulation variation in the dietary habits of snakes. Web Ecol. 2006, 6, 2–16. [Google Scholar] [CrossRef]
- Margres, M.J.; McGivern, J.J.; Seavy, M.; Wray, K.P.; Facente, J.; Rokyta, D.R. Contrasting modes and tempos of venom expression evolution in two snake species. Genetics 2015, 199, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Secor, S.M.; Nagy, K.A. Bioenergetic Correlates of Foraging Mode for the Snakes Crotalus cerastes and Masticophis flagellum. Ecology 1994, 75, 1600–1614. [Google Scholar] [CrossRef]
- Secor, S.M. Ecological Aspects of Foraging Mode for the Snakes Crotalus cerastes and Masticophis flagellum. Herpetol. Monogr. 1995, 9, 169–186. [Google Scholar] [CrossRef]
- Salomão, M.D.; Santos, S.M.A.; Guiseppe, P. Activity pattern of Crotalus durissus (Viperidae, Crotalinae): Feeding, reproduction and snakebite. Stud. Neotrop. Fauna Environ. 1995, 30, 101–106. [Google Scholar] [CrossRef]
- Sant’Anna, S.S.; Abe, A.S. Diet of the rattlesnake Crotalus durissus in southeastern Brazil (Serpentes, Viperidae). Stud. Neotrop. Fauna Environ. 2007, 42, 169–174. [Google Scholar] [CrossRef]
- Meik, J.M.; Schaack, S.; Ingrasci, M.J.; Lawing, A.M. Notes on activity, body size variation, and diet in insular speckled rattlesnakes from the western Sea of Cortés, Mexico. Herpetol. Rev. 2012, 43, 556–560. [Google Scholar]
- Mackessy, S.P.; Kwame, W.; Ashton, K.G. Ontogenetic Variation in Venom Composition and Diet of Crotalus oreganus concolor: A Case of Venom Paedomorphosis? Copeia 2003, 2003, 769–782. [Google Scholar] [CrossRef]
- Hayes, W.K. Prey-Handling and Envenomation Strategies of Prairie Rattlesnakes (Crotalus v. viridis) Feeding on Mice and Sparrows. J. Herpetol. 1992, 26, 496–499. [Google Scholar] [CrossRef]
- Voss, R.S. Opossums (Mammalia: Didelphidae) in the diets of Neotropical pitvipers (Serpentes: Crotalinae): Evidence for alternative coevolutionary outcomes? Toxicon 2013, 66, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Akira, M.; Mamoru, T. Feeding Characteristics of a Japanese Pitviper, Ovophis okinavensis, on Okinawa Island: Seasonally Biased but Ontogenetically Stable Exploitation on Small Frogs. Curr. Herpetol. 2011, 30, 41–52. [Google Scholar] [CrossRef]
- Chijiwa, T.; Yamaguchi, Y.; Ogawa, T.; Deshimaru, M.; Nobuhisa, I.; Nakashima, K.; Oda-Ueda, N.; Fukumaki, Y.; Hattori, S.; Ohno, M. Interisland evolution of Trimeresurus flavoviridis venom phospholipase A(2) isozymes. J. Mol. Evol. 2003, 56, 286–293. [Google Scholar] [CrossRef] [PubMed]


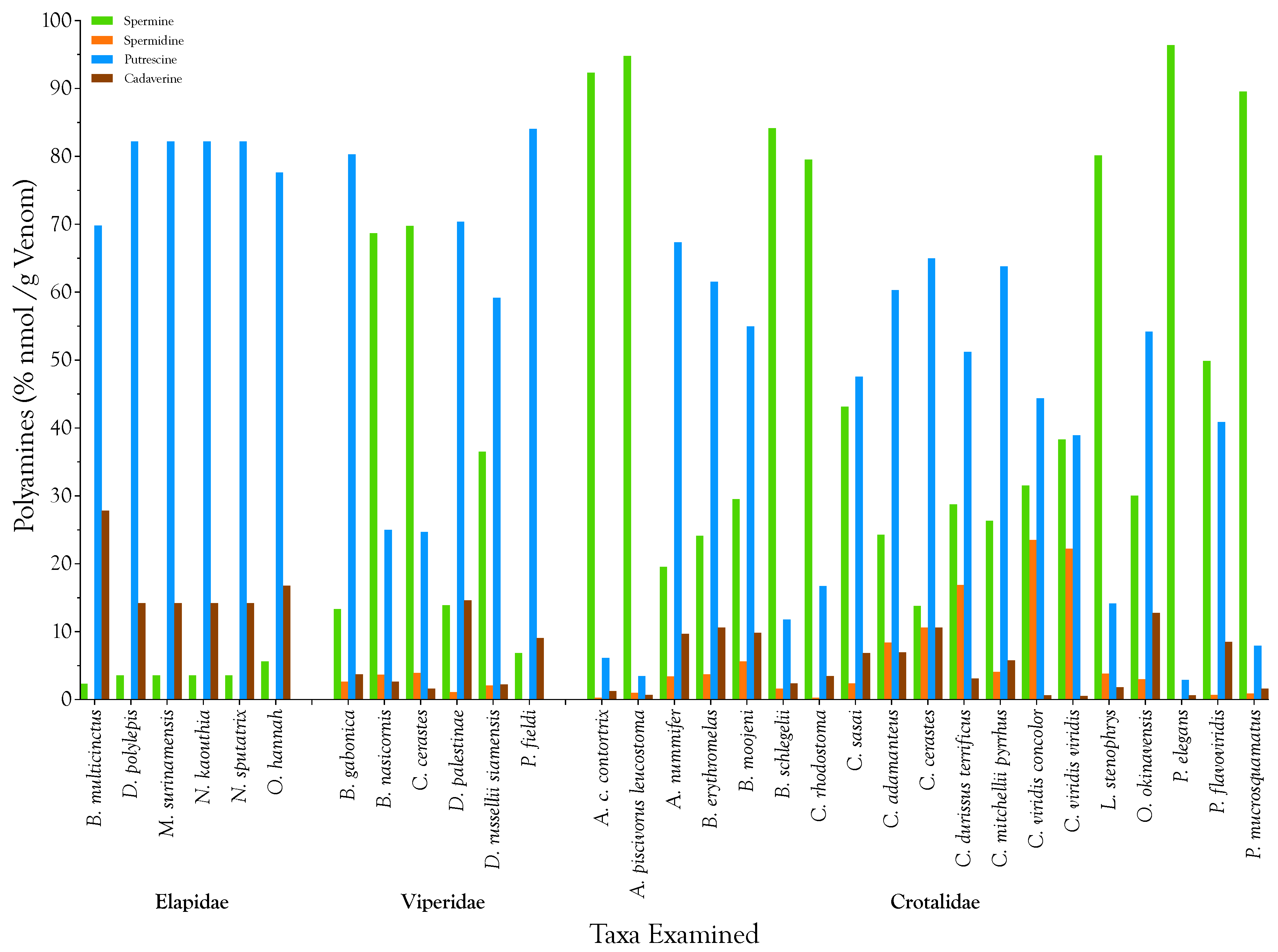
| Snake Taxa | Venom Polyamines (µg/g) | Venom Polyamines (nmol/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spermine | Spermidine | Putrescine | Cadaverine | Total | Spermine | Spermidine | Putrescine | Cadaverine | Total | |
| Elapidae | ||||||||||
| B. multicinctus | 0.006 | 0.000 | 0.083 | 0.038 | 0.128 | 0.032 | 0.000 | 0.944 | 0.376 | 1 |
| D. polylepis | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| M. surinamensis | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| N. kaouthia | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| N. sputatrix | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| O. hannah | 0.013 | 0.000 | 0.077 | 0.019 | 0.109 | 0.063 | 0.000 | 0.871 | 0.188 | 1 |
| Mean | 0.007 | 0.000 | 0.069 | 0.018 | 0.095 | 0.037 | 0.000 | 0.787 | 0.177 | 1 |
| Std. Deviation | 0.003 | 0.000 | 0.009 | 0.010 | 0.019 | 0.013 | 0.000 | 0.097 | 0.100 | 0 |
| Viperidae | ||||||||||
| B. gabonica | 1.178 | 0.166 | 3.085 | 0.166 | 4.595 | 5.820 | 1.146 | 34.995 | 1.628 | 44 |
| B. nasicornis | 81.158 | 3.104 | 12.864 | 1.568 | 98.694 | 401.099 | 21.370 | 145.933 | 15.345 | 584 |
| C. cerastes | 246.477 | 9.971 | 38.003 | 2.899 | 297.350 | 1218.132 | 68.649 | 431.117 | 28.373 | 1746 |
| D. palestinae | 2.835 | 0.160 | 6.259 | 1.504 | 10.758 | 14.012 | 1.102 | 71.006 | 14.719 | 101 |
| D. r. siamensis | 50.816 | 2.080 | 35.866 | 1.555 | 90.317 | 251.142 | 14.320 | 406.870 | 15.220 | 688 |
| P. fieldi | 0.019 | 0.000 | 0.102 | 0.013 | 0.134 | 0.095 | 0.000 | 1.162 | 0.125 | 1 |
| Mean | 63.747 | 2.580 | 16.030 | 1.284 | 83.642 | 315.050 | 17.764 | 181.847 | 12.569 | 527 |
| Std. Deviation | 95.443 | 3.834 | 16.751 | 1.066 | 113.557 | 471.697 | 26.395 | 190.031 | 10.428 | 664 |
| Crotalidae | ||||||||||
| A. c. contortrix | 220.019 | 0.448 | 6.381 | 1.523 | 228.371 | 1087.347 | 3.084 | 72.386 | 14.907 | 1178 |
| A. p. leucostoma | 80.627 | 0.627 | 1.280 | 0.307 | 82.842 | 398.474 | 4.318 | 14.521 | 3.006 | 420 |
| A. nummifer | 0.051 | 0.006 | 0.077 | 0.013 | 0.147 | 0.253 | 0.044 | 0.871 | 0.125 | 1 |
| B. erythromelas | 0.058 | 0.006 | 0.064 | 0.013 | 0.141 | 0.285 | 0.044 | 0.726 | 0.125 | 1 |
| B. moojeni | 0.608 | 0.083 | 0.493 | 0.102 | 1.286 | 3.005 | 0.573 | 5.590 | 1.002 | 10 |
| B. schlegelii | 104.467 | 1.466 | 6.374 | 1.504 | 113.811 | 516.295 | 10.090 | 72.313 | 14.719 | 613 |
| C. rhodostoma | 67.962 | 0.154 | 6.227 | 1.498 | 75.840 | 335.878 | 1.057 | 70.643 | 14.656 | 422 |
| C. sasai | 0.160 | 0.006 | 0.077 | 0.013 | 0.256 | 0.791 | 0.044 | 0.871 | 0.125 | 2 |
| C. adamanteus | 10.822 | 2.688 | 11.712 | 1.568 | 26.790 | 53.486 | 18.506 | 132.864 | 15.345 | 220 |
| C. cerastes | 3.885 | 2.144 | 7.974 | 1.510 | 15.514 | 19.199 | 14.761 | 90.464 | 14.782 | 139 |
| C. d. terrificus | 30.598 | 12.890 | 23.725 | 1.670 | 68.883 | 151.223 | 88.741 | 269.141 | 16.348 | 525 |
| C. m. pyrrhus | 0.115 | 0.013 | 0.122 | 0.013 | 0.262 | 0.569 | 0.088 | 1.379 | 0.125 | 2 |
| C. v. concolor | 1.984 | 1.062 | 1.216 | 0.019 | 4.282 | 9.805 | 7.314 | 13.795 | 0.188 | 31 |
| C. v. viridis | 2.972 | 1.238 | 1.317 | 0.022 | 5.549 | 14.687 | 8.524 | 14.942 | 0.216 | 38 |
| L. stenophrys | 26.240 | 0.902 | 2.022 | 0.301 | 29.466 | 129.683 | 6.213 | 22.943 | 2.944 | 162 |
| O. okinavensis | 0.090 | 0.006 | 0.070 | 0.019 | 0.186 | 0.443 | 0.044 | 0.799 | 0.188 | 1 |
| P. elegans | 469.459 | 0.179 | 6.214 | 1.504 | 477.357 | 2320.150 | 1.234 | 70.498 | 14.719 | 2407 |
| P. flavoviridis | 17.376 | 0.173 | 6.208 | 1.498 | 25.254 | 85.875 | 1.190 | 70.425 | 14.656 | 172 |
| P. mucrosquamatus | 1602.214 | 11.315 | 61.747 | 14.963 | 1690.240 | 7918.426 | 77.902 | 700.479 | 146.440 | 8843 |
| Mean | 138.932 | 1.864 | 7.542 | 1.477 | 149.815 | 686.626 | 12.830 | 85.561 | 14.454 | 799 |
| Std. Deviation | 372.175 | 3.699 | 14.341 | 3.346 | 390.393 | 1839.355 | 25.468 | 162.691 | 32.745 | 2033 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aird, S.D.; Villar Briones, A.; Roy, M.C.; Mikheyev, A.S. Polyamines as Snake Toxins and Their Probable Pharmacological Functions in Envenomation. Toxins 2016, 8, 279. https://doi.org/10.3390/toxins8100279
Aird SD, Villar Briones A, Roy MC, Mikheyev AS. Polyamines as Snake Toxins and Their Probable Pharmacological Functions in Envenomation. Toxins. 2016; 8(10):279. https://doi.org/10.3390/toxins8100279
Chicago/Turabian StyleAird, Steven D., Alejandro Villar Briones, Michael C. Roy, and Alexander S. Mikheyev. 2016. "Polyamines as Snake Toxins and Their Probable Pharmacological Functions in Envenomation" Toxins 8, no. 10: 279. https://doi.org/10.3390/toxins8100279