The Role of Botulinum Toxin Type A in the Clinical Management of Refractory Anterior Knee Pain
Abstract
:1. Anterior Knee Pain
1.1. Pathophysiology of Anterior Knee Pain
1.2. Management of Anterior Knee Pain
1.3. Refractory Anterior Knee Pain
2. Botulinum Toxin Type A (BoNT-A)
2.1. Clinical Uses of BoNT-A
2.2. The Role of BoNT-A in Managing AKP
2.3. Clinical Trials of Safety and Efficacy of BoNT in AKP



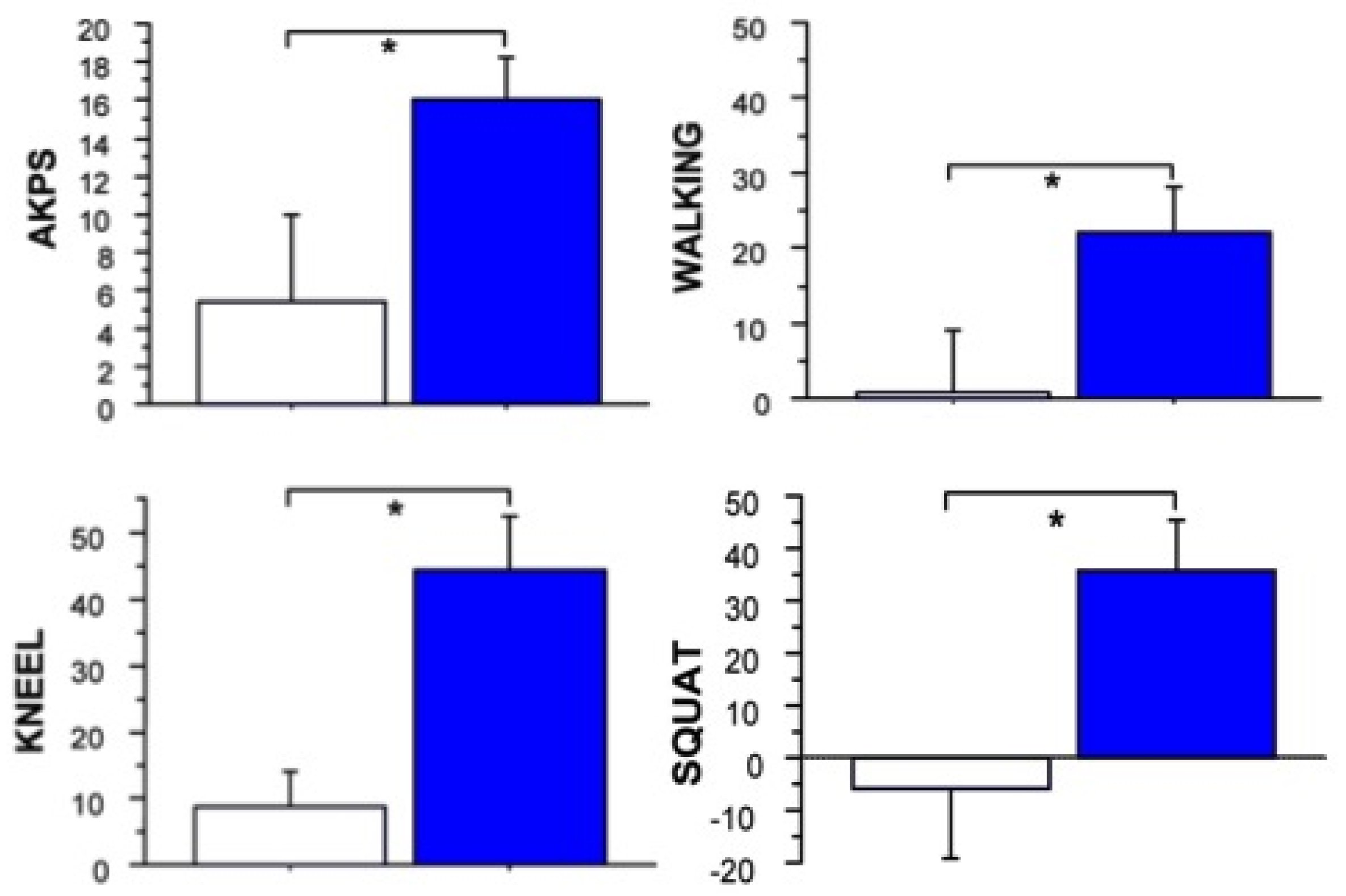
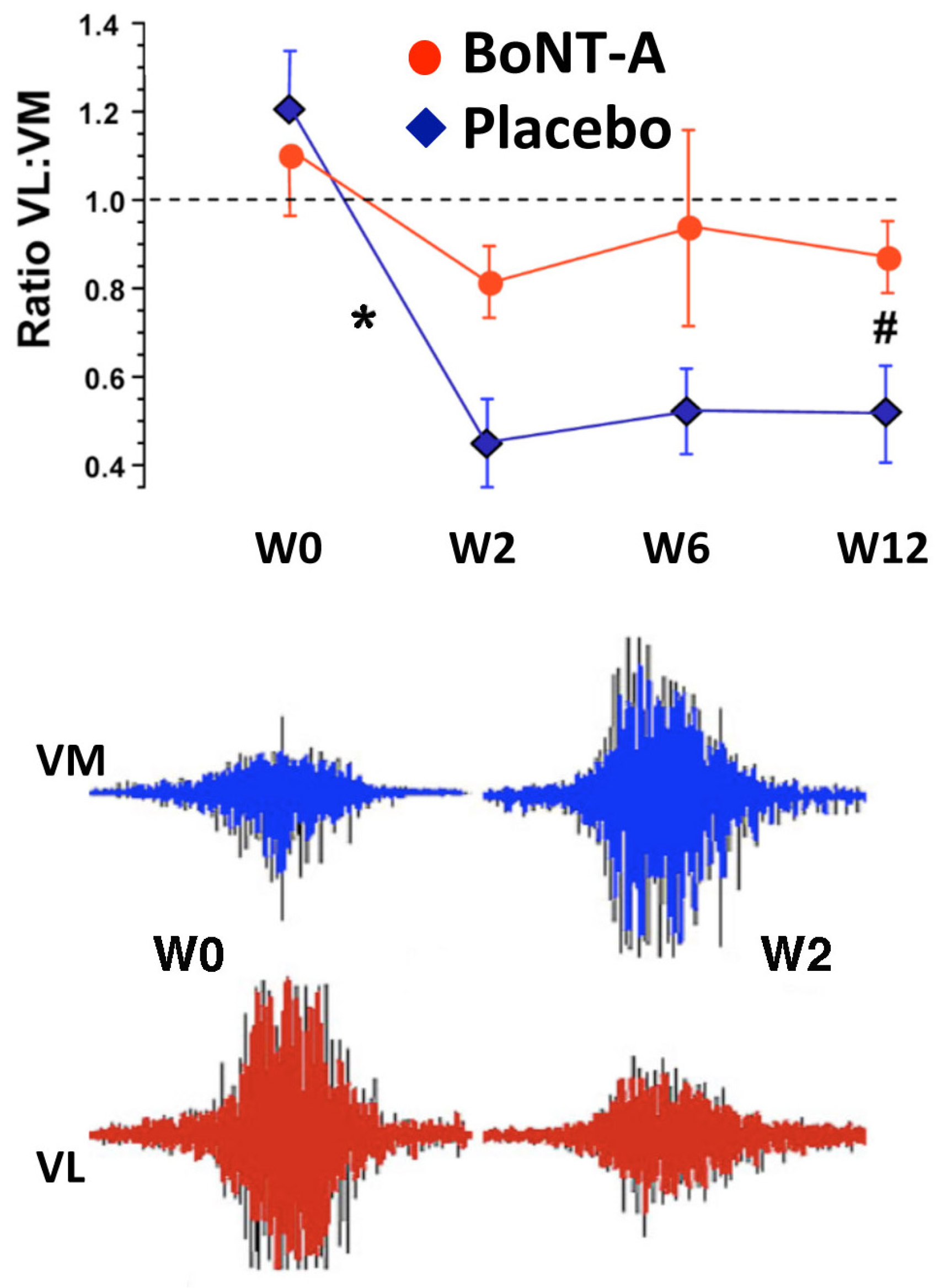
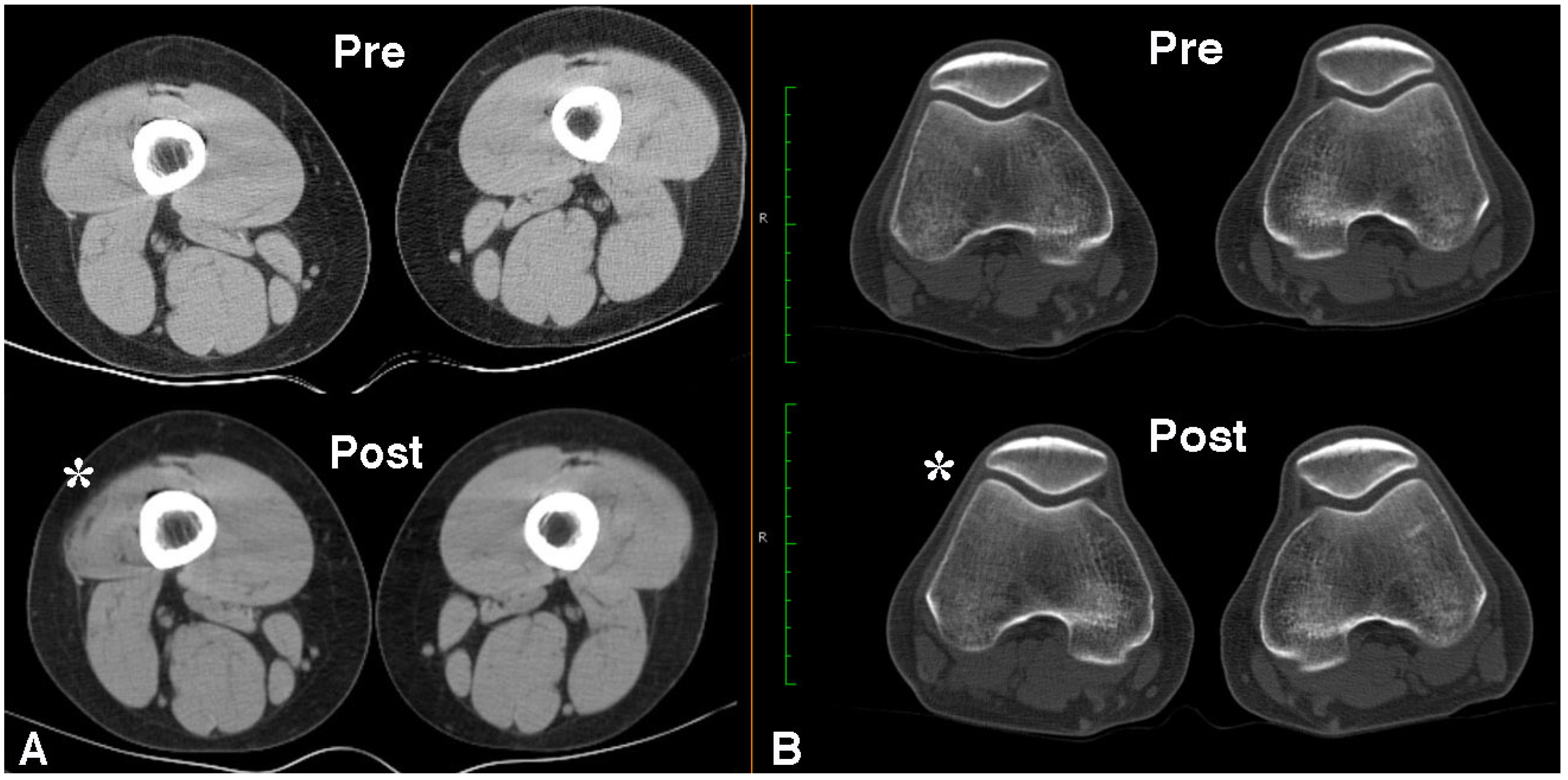
2.4. Long Term Effects of BoNT-A Injection in AKP
2.5. Possible Mechanisms of Effect of BoNT-A Injection in AKP
2.6. Clinical Algorithm for the Use of BoNT-A in AKP
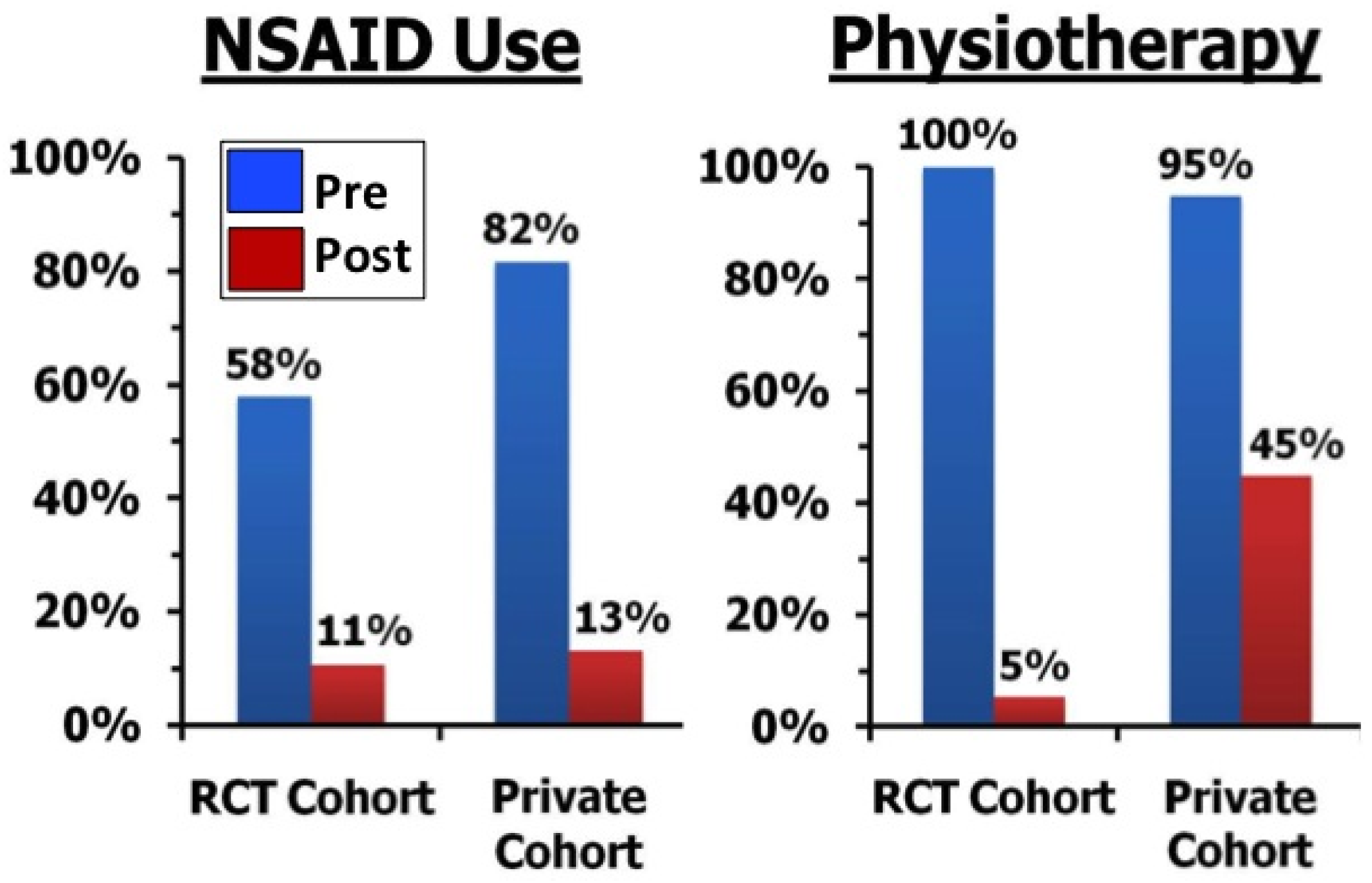
2.7. Future Research
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R.; Zumbo, B.D. A retrospective case-control analysis of 2002 running injuries. Br. J. Sports Med. 2002, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Jaakkola, L.H.; Koskinen, S.K.; Taimela, S.; Hurme, M.; Nelimarkka, O. Scoring of patellofemoral disorders. Arthroscopy 1993, 9, 159–163. [Google Scholar] [CrossRef]
- Teitge, R.A. Patellofemoral syndrome a paradigm for current surgical strategies. Orthop. Clin. N. Am. 2008, 39, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.R.; Curtis Bay, R. Prevalence of anterior knee pain in 18–35 year-old females. Int. J. Sports Phys. Ther. 2012, 7, 396–401. [Google Scholar] [PubMed]
- Lonner, J.H. Patellofemoral arthroplasty. Orthopedics 2010, 33, 653. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Wood, L.; Selfe, J.; Peat, G. Anterior knee pain in younger adults as a precursor to subsequent patellofemoral osteoarthritis: A systematic review. BMC Musculoskelet. Disord. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, L.A.; Kerslake, S.; Irving, C. Anterior knee pain in the athlete. Clin. Sports Med. 2014, 33, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, E.; Werner, S.; Mikkelsen, C.; Van Tiggelen, D.; Vanden Berghe, L.; Cerulli, G. Clinical classification of patellofemoral pain syndrome: Guidelines for non-operative treatment. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Werner, S. Anterior knee pain: An update of physical therapy. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Hayes, K.C.; Alexander, I.J. Knee joint effusion and quadriceps reflex inhibition in man. Arch. Phys. Med. Rehabil. 1984, 65, 171–177. [Google Scholar] [PubMed]
- Natri, A.; Kannus, P.; Järvinen, M. Which factors predict the long term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow up study. Med. Sci. Sports Exerc. 1998, 30, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Chester, R.; Smith, T.O.; Sweeting, D.; Dixon, J.; Wood, S.; Song, F. The relative timing of VMO and VL in the aetiology of anterior knee pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tiggelen, D.; Cowan, S.; Coorevits, P.; Duvigneaud, N.; Witvrouw, E. Delayed vastus medialis obliquus to vastus lateralis onset timing contributes to the development of patellofemoral pain in previously healthy men: A prospective study. Am. J. Sports Med. 2009, 37, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chien, C.C.; Wu, S.K.; Liau, J.J.; Jan, M.H. Electromechanical delay of the vastus medialis obliquus and vastus lateralis in individuals with patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2012, 42, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, J.P.; Shea, K.P. Mechanical basis for patellofemoral pain and cartilage breakdown. In Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy; Ewing, J.W., Ed.; Raven Press: New York, NY, USA, 1990; pp. 93–101. [Google Scholar]
- Pal, S.; Draper, C.E.; Fredericson, M.; Gold, G.E.; Delp, S.L.; Beaupre, G.S.; Besier, T.F. Patellar maltracking correlates with vastus medialis activation delay in patellofemoral pain patients. Am. J. Sports Med. 2011, 39, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Besier, T.F.; Draper, C.E.; Fredericson, M.; Gold, G.E.; Beaupre, G.S.; Delp, S.L. Patellar tilt correlates with vastus lateralis: Vastus medialis activation ratio in maltracking patellofemoral pain patients. J. Orthop. Res. 2012, 30, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.L.; Willson, J.D.; Ballantyne, B.T.; Davis, I.M. Hip strength in females with and without patellofemoral pain. J. Orthop. Sports Phys. Ther. 2003, 33, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: A theoretical perspective. J. Orthop. Sports Phys. Ther. 2003, 33, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.J.; Lack, S.; Malliaras, P.; Morrissey, D. Gluteal muscle activity and patellofemoral pain syndrome: A systematic review. Br. J. Sports Med. 2013, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.R.; van der Wurff, P. Females with patellofemoral pain syndrome have weak hip muscles: A systematic review. Aust. J. Physiother. 2009, 55, 9–15. [Google Scholar] [CrossRef]
- Rathleff, M.S.; Rathleff, C.R.; Crossley, K.M.; Barton, C.J. Is hip strength a risk factor for patellofemoral pain? A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1088. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Strickland, S.M. Patellofemoral pain: An update on diagnostic and treatment options. Curr. Rev. Musculoskelet. Med. 2013, 6, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Swart, N.M.; van Linschoten, R.; Bierma-Zeinstra, S.M.; van Middelkoop, M. The additional effect of orthotic devices on exercise therapy for patients with patellofemoral pain syndrome: A systematic review. Br. J. Sports Med. 2012, 46, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.J.; Selfe, J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst. Rev. 2012, 4. [Google Scholar] [CrossRef]
- Draper, C.E.; Besier, T.F.; Santos, J.M.; Jennings, F.; Fredericson, M.; Gold, G.E.; Beaupre, G.S.; Delp, S.L. Using real-time MRI to quantify altered joint kinematics in subjects with patellofemoral pain and to evaluate the effects of a patellar brace or sleeve on joint motion. J. Orthop. Res. 2009, 27, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Arvidsson, H.; Arvidsson, I.; Eriksson, E. Electrical stimulation of vastus medialis and stretching of lateral thigh muscles in patients with patello-femoral symptoms. Knee Surg. Sports Traumatol. Arthrosc. 1993, 1, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.B.; Powers, C.M. Predictors of hip internal rotation during running: An evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am. J. Sports Med. 2009, 37, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M.; Ward, S.R.; Chan, L.D.; Chen, Y.J.; Terk, M.R. The effect of bracing on patella alignment and patellofemoral joint contact area. Med. Sci. Sports Exerc. 2004, 36, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M.; Doubleday, K.L.; Escudero, C. Influence of patellofemoral bracing on pain, knee extensor torque, and gait function in females with patellofemoral pain. Physiother. Theory Pract. 2008, 24, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.A.; Mazahery, B.T.; Koh, J.L.; Zhang, L.Q. Effect of bracing on dynamic patellofemoral contact mechanics. J. Rehabil. Res. Dev. 2010, 47, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.J.; Bisset, L.M.; Crossley, K.M.; Vicenzino, B. Efficacy of nonsurgical interventions for anterior knee pain: Systematic review and meta-analysis of randomized trials. Sports Med. 2012, 42, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, R.A.; Lankhorst, N.E.; van Linschoten, R.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst. Rev. 2015, 1. [Google Scholar] [CrossRef]
- Lack, S.; Barton, C.; Vicenzino, B.; Morrissey, D. Outcome predictors for conservative patellofemoral pain management: A systematic review and meta-analysis. Sports Med. 2014, 44, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Stathopulu, E.; Baildam, E. Anterior knee pain: A long term follow up. Rheumatology (Oxford) 2003, 42, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Rathleff, M.S.; Rasmussen, S.; Olesen, J.L. Unsatisfactory long-term prognosis of conservative treatment of patellofemoral pain syndrome. Ugeskr. Laeger 2012, 174, 1008–1013. [Google Scholar] [PubMed]
- Smith, T.O.; Song, F.; Donell, S.T.; Hing, C.B. Operative versus non-operative management of patellar dislocation. A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 988–998. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, A.; Meunier, F.A.; Molgo, J.; Aoki, K.R.; Dolly, J.O. Functional repair of motor endplates after botulinum toxin type A poisoning. Proc. Natl. Acad. Sci. USA 1999, 96, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D. Therapeutic use of botulinum toxin in neurorehabilitation. J. Toxicol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Guettard, E.; Roze, E.; Abada, G.; Lemesle, C.; Vidailhet, M.; Laurent-Vannier, A.; Chevignard, M.P. Management of spasticity and dystonia in children with acquired brain injury with rehabilitation and botulinum toxin A. Dev. Neurorehabil. 2009, 12, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.P.; Singer, B.J. The role of botulinum toxin injection in achieving balanced muscle function. Disabil. Rehabil. 2007, 29, 1759–1760. [Google Scholar] [CrossRef]
- Jabbari, B.; Machado, D. Treatment of refractory pain with botulinum toxins—An evidence-based review. Pain Med. 2011, 12, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.J.; Silbert, P.L.; Dunne, J.W.; Song, S.; Singer, K.P. An open label pilot investigation of the efficacy of Botulinum toxin type A [Dysport] injection in the rehabilitation of chronic anterior knee pain. Disabil. Rehabil. 2006, 28, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.F.; Pidcoe, P.E.; Ericksen, J. Botulinum toxin type A for nonsurgical lateral release in patellofemoral pain syndrome: A case study. Mil. Med. 2011, 176, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.J.; Silbert, P.L.; Dunne, J.W.; Song, S.; Singer, K.P. Treatment of refractory anterior knee pain using botulinum toxin type A (Dysport) injection to the distal vastus lateralis muscle: A randomised placebo controlled crossover trial. Br. J. Sports Med. 2011, 45, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Silbert, B.I.; Singer, B.J.; Silbert, P.L.; Gibbons, J.T.; Singer, K.P. Enduring efficacy of Botulinum toxin type A injection for refractory anterior knee pain. Disabil. Rehabil. 2012, 34, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Tang, A.C.; Lin, S.C.; Tang, S.F. Anterior knee pain caused by patellofemoral pain syndrome can be relieved by Botulinum toxin type A injection. Clin. Neurol. Neurosurg. 2015, 129 (Suppl. S1), S27–S29. [Google Scholar] [CrossRef]
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. J. Pediatr. Ophthalmol. 1980, 17, 21–25. [Google Scholar] [CrossRef]
- Botter, A.; Oprandi, G.; Lanfranco, F.; Allasia, S.; Maffiuletti, N.A.; Minetto, M.A. Atlas of the muscle motor points for the lower limb: Implications for electrical stimulation procedures and electrode positioning. Eur. J. Appl. Physiol. 2011, 111, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Kuriki, H.U.; Silva, C.R.; Alves, N.; Mícolis de Azevedo, F. Diagnostic accuracy of the electromyography parameters associated with anterior knee pain in the diagnosis of patellofemoral pain syndrome. Arch. Phys. Med. Rehabil. 2014, 95, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; Bennell, K.L.; Cowan, S.M.; Green, S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch. Phys. Med. Rehabil. 2004, 85, 815–822. [Google Scholar] [CrossRef]
- Watson, C.J.; Propps, M.; Ratner, J.; Zeigler, D.L.; Horton, P.; Smith, S.S. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J. Orthop. Sports Phys. Ther. 2005, 35, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Beaman, D.N.; Glover, R.A.; Janda, D. Innervation of the human knee joint by substance-P fibers. Arthroscopy 1990, 6, 254–263. [Google Scholar] [CrossRef]
- Telleria-Diaz, A.; Ebersberger, A.; Vasquez, E.; Schache, F.; Kahlenbach, J.; Schaible, H.G. Different effects of spinally applied prostaglandin D2 on responses of dorsal horn neurons with knee input in normal rats and in rats with acute knee inflammation. Neuroscience 2008, 156, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Rapp, D.E.; Turk, K.W.; Bales, G.T.; Cook, S.P. Botulinum toxin type A inhibits calcitonin gene-related peptide release from isolated rat bladder. J. Urol. 2006, 175, 1138–1142. [Google Scholar] [CrossRef]
- Birklein, F.; Schmelz, M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci. Lett. 2008, 437, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, J.C. Possible mechanisms for the effects of botulinum toxin on pain. Clin. J. Pain 2002, 18, S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G. Botulinum toxin for the treatment of musculoskeletal pain and spasm. Curr. Pain Headache Rep. 2002, 6, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005, 26, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Blersch, W.; Schulte-Mattler, W.J.; Przywara, S.; May, A.; Bigalke, H.; Wohlfarth, K. Botulinum toxin A and the cutaneous nociception in humans: A prospective, double-blind, placebo-controlled, randomized study. J. Neurol. Sci. 2002, 205, 59–63. [Google Scholar] [CrossRef]
- Voller, B.; Sycha, T.; Gustorff, B.; Schmetterer, L.; Lehr, S.; Eichler, H.G.; Auff, E.; Schnider, P. A randomized, double-blind, placebo controlled study on analgesic effects of botulinum toxin A. Neurology 2003, 61, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Sycha, T.; Samal, D.; Chizh, B.; Lehr, S.; Gustorff, B.; Schnider, P.; Auff, E. A lack of antinociceptive or antiinflammatory effect of botulinum toxin A in an inflammatory human pain model. Anesth. Analg. 2006, 102, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Mattler, W.J.; Opatz, O.; Blersch, W.; May, A.; Bigalke, H.; Wohlfahrt, K. Botulinum toxin A does not alter capsaicin-induced pain perception in human skin. J. Neurol. Sci. 2007, 260, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.Y.; Sheu, J.J.; Yu, J.M.; Chen, W.T.; Tseng, I.J.; Chang, H.H.; Hu, C.J. Botulinum toxin for diabetic neuropathic pain: A randomized double-blind crossover trial. Neurology 2009, 72, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Shaari, C.M.; George, E.; Wu, B.L.; Biller, H.F.; Sanders, I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope 1991, 101, 960–964. [Google Scholar] [CrossRef] [PubMed]
- George, E.F.; Zimbler, M.; Wu, B.L.; Biller, H.F.; Sanders, I. Quantitative mapping of the effect of botulinum toxin injections in the thyroarytenoid muscle. Ann. Otol. Rhinol. Laryngol. 1992, 101, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A. Use of botulinum toxin in musculoskeletal pain. F1000Research 2013, 2, 52. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, B.J.; Silbert, B.I.; Silbert, P.L.; Singer, K.P. The Role of Botulinum Toxin Type A in the Clinical Management of Refractory Anterior Knee Pain. Toxins 2015, 7, 3388-3404. https://doi.org/10.3390/toxins7093388
Singer BJ, Silbert BI, Silbert PL, Singer KP. The Role of Botulinum Toxin Type A in the Clinical Management of Refractory Anterior Knee Pain. Toxins. 2015; 7(9):3388-3404. https://doi.org/10.3390/toxins7093388
Chicago/Turabian StyleSinger, Barbara J., Benjamin I. Silbert, Peter L. Silbert, and Kevin P. Singer. 2015. "The Role of Botulinum Toxin Type A in the Clinical Management of Refractory Anterior Knee Pain" Toxins 7, no. 9: 3388-3404. https://doi.org/10.3390/toxins7093388




