Murine Anorectic Response to Deoxynivalenol (Vomitoxin) Is Sex-Dependent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study 1
Feed Refusal upon Acute DON Exposure Is Greater in Males than Females
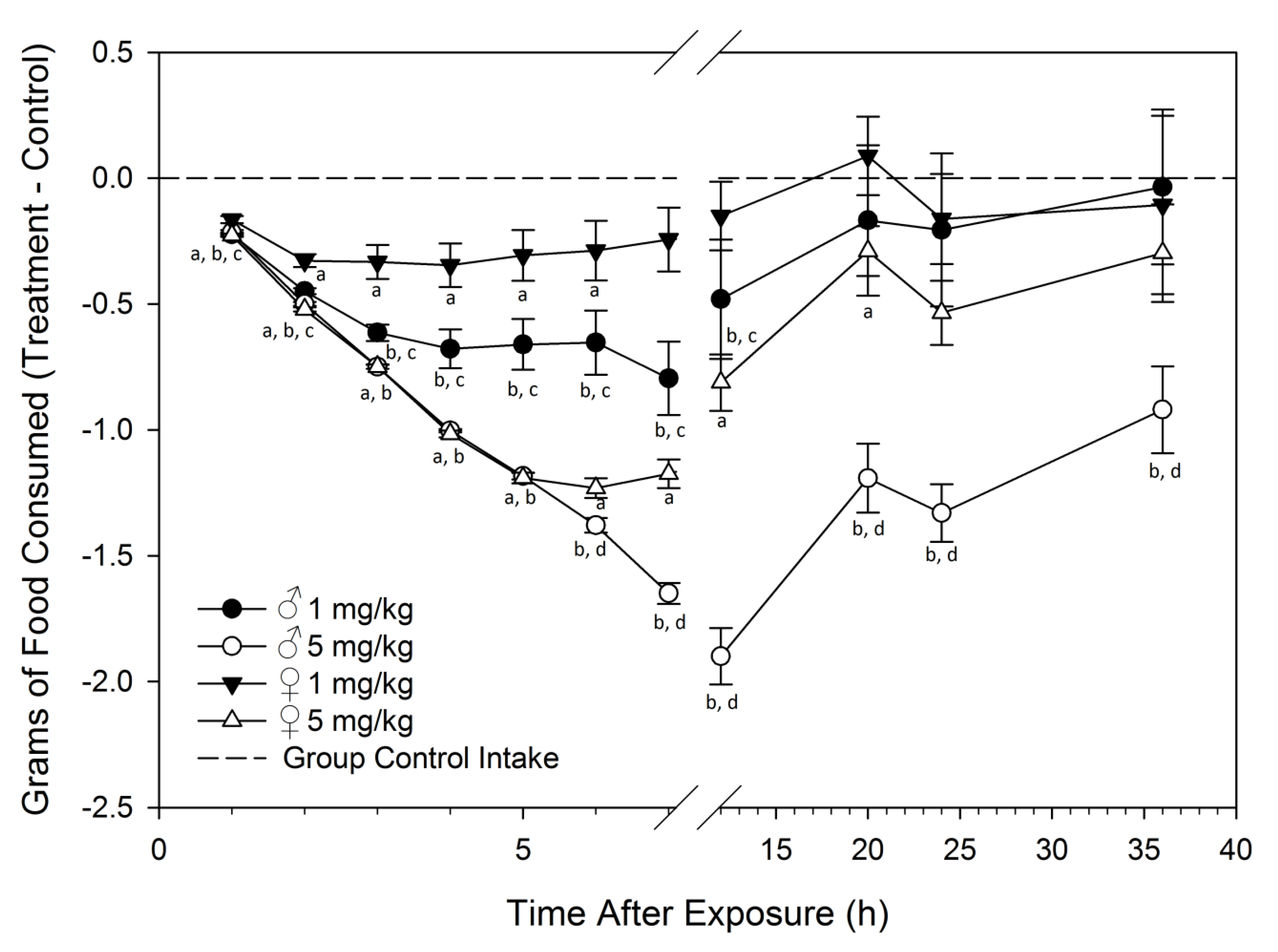
2.2. Study 2
2.2.1. DON Organ Concentrations Are Higher in Male Mice after Acute DON Exposure
| Organ | DON Equivalents (nmol/g) | |||
|---|---|---|---|---|
| 1 h | 4 h | |||
| ♂ | ♀ | ♂ | ♀ | |
| Kidney | 3.85 ± 0.3 * | 2.21 ± 0.2 | 0.30 ± 0.03 * | 0.17 ± 0.01 |
| Liver | 2.60 ± 0.2 p = 0.09 | 1.86 ± 0.3 | 0.36 ± 0.1 * | 0.14 ± 0.02 |
| Plasma | 2.72 ± 0.2 | 2.33 ± 0.2 | 0.20 ± 0.01 * | 0.12 ± 0.01 |
| Heart | 2.24 ± 0.1 * | 1.60 ± 0.2 | 0.19 ± 0.01 * | 0.11 ± 0.01 |
| Spleen | 1.44 ± 0.1 | 1.16 ± 0.2 | 0.17 ± 0.01 * | 0.10 ± 0.01 |
| Brain | 0.61 ± 0.01 | 0.64 ± 0.04 | 0.33 ± 0.02 | 0.30 ± 0.05 |
2.2.2. Plasma IL-6 Is Higher in Male Mice than Female Mice upon Acute DON Exposure
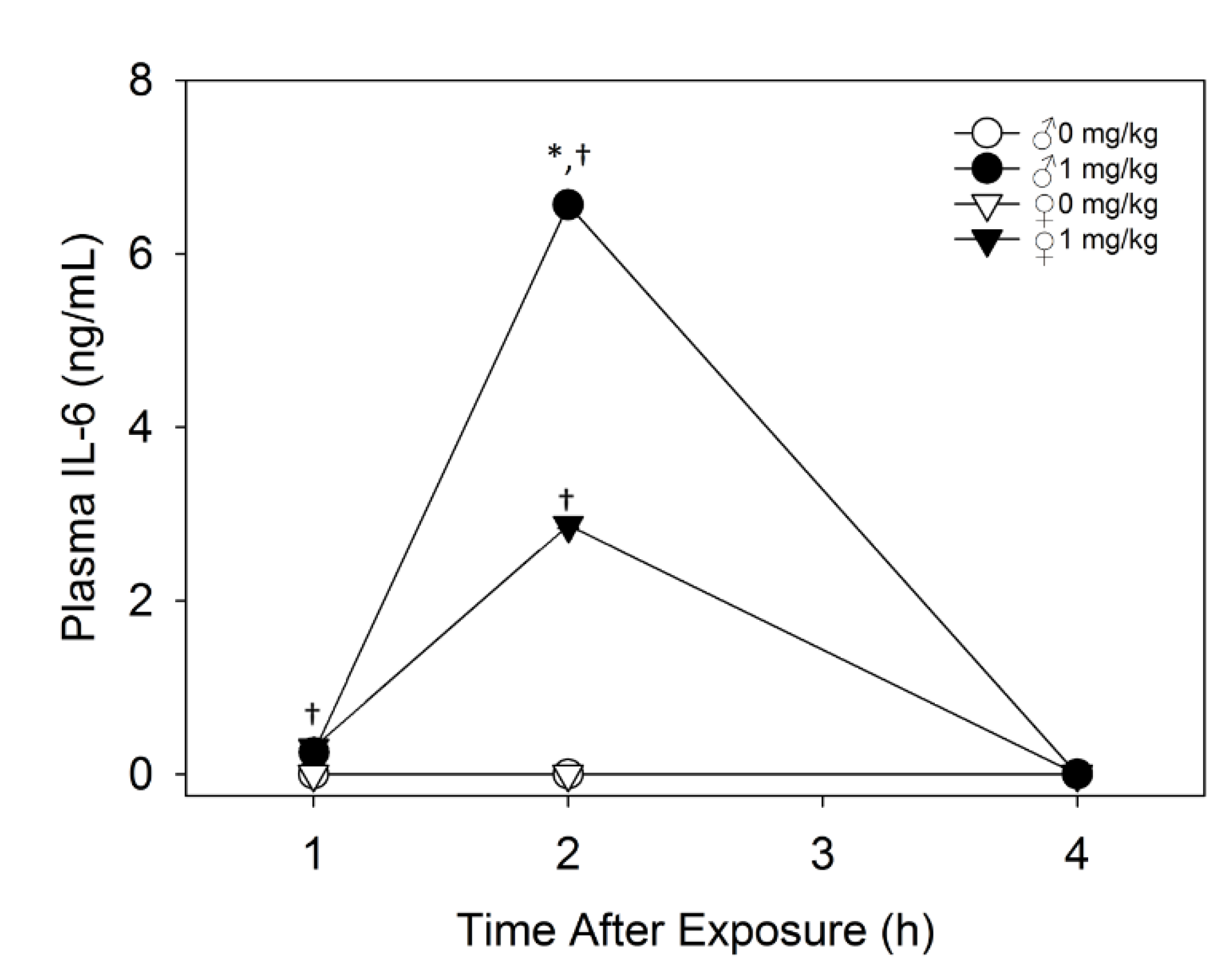
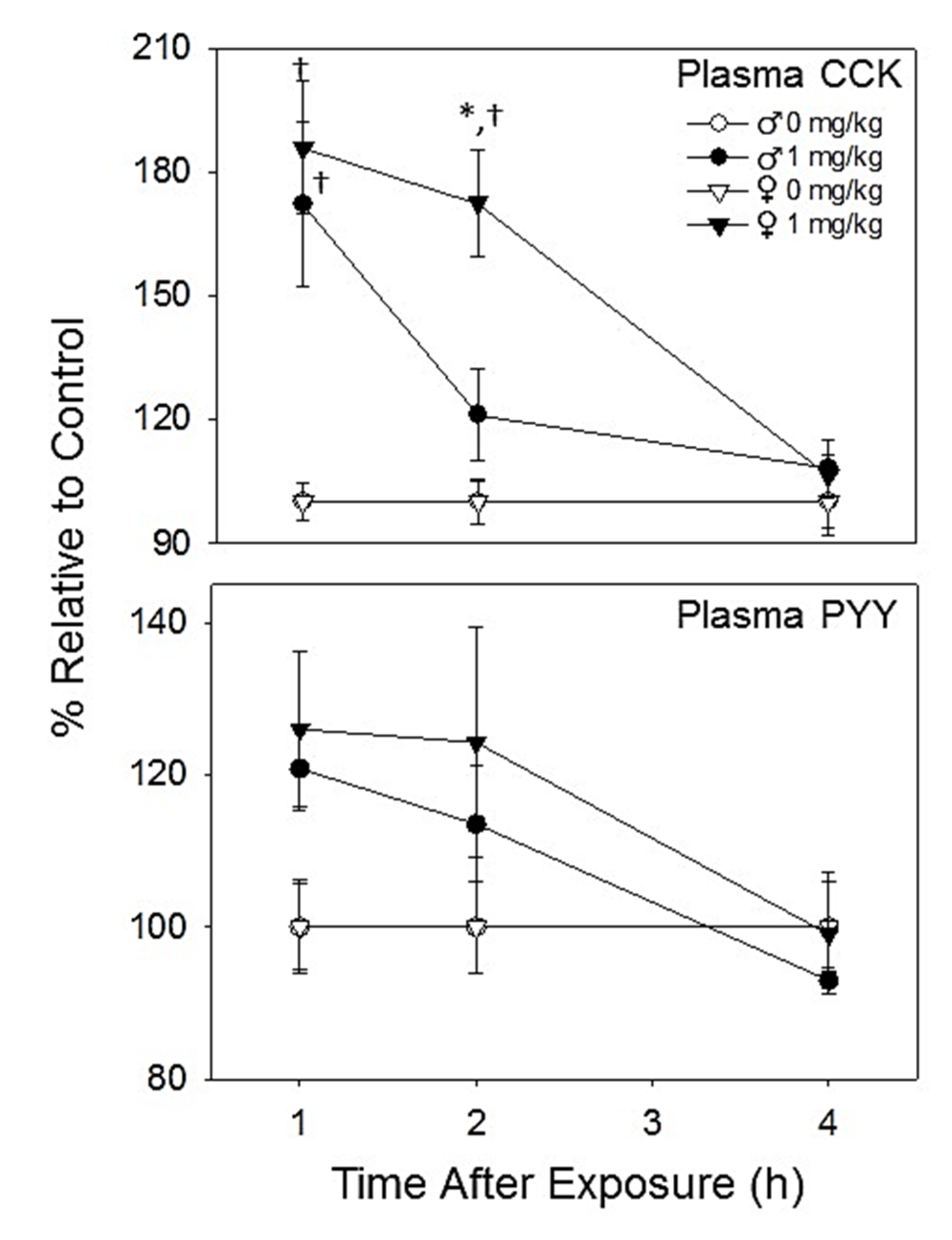
2.2.3. Plasma CCK and PYY Are Increased in Both Female and Male Mice with DON Exposure
2.3. Study 3
2.3.1. Initial Food Intake Is Suppressed in Male Mice But Not Females Exposed to Dietary DON
| Group | % Control Food Intake 0–24 h | % Control Food Intake 24–48 h |
|---|---|---|
| ♂ 1 ppm | 99.5 ± 3.8 * | 95.2 ± 7.2 * |
| ♂ 2.5 ppm | 85.7 ± 7.3 * | 85.0 ± 4.9 * |
| ♂ 10 ppm | 56.9 ± 10.2 * | 74.9 ± 5.6 * |
| ♀ 1 ppm | 100.7 ± 4.2 | 95.6 ± 4.6 |
| ♀ 2.5 ppm | 99.5 ± 8.1 | 101.4 ± 5.9 |
| ♀ 10 ppm | 84.0 ± 7.8 | 92.3 ± 8.0 |
2.3.2. Dietary DON Exposure Causes Greater Suppression of Weight Gain in Male Mice

| Treatment | Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| ♂ 1 ppm | - | - | - | - | - | - | - | - | a, b |
| ♂ 2.5 ppm | - | - | - | - | - | - | - | - | a, b |
| ♂ 10 ppm | - | - | - | a, b | a, b | a, b | a, b | a, b | a, b |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| ♂ 1 ppm | a | a, b | - | a, b | a | - | - | a, b | a |
| ♂ 2.5 ppm | a, b | a, b | - | a, b | - | - | - | - | - |
| ♂ 10 ppm | a, b | a, b | a, b | a, b | a, b | a, b | a, b | a, b | a, b |
2.3.3. Dietary DON Exposure Contributes to Elevated Liver DON Equivalents in Male Mice
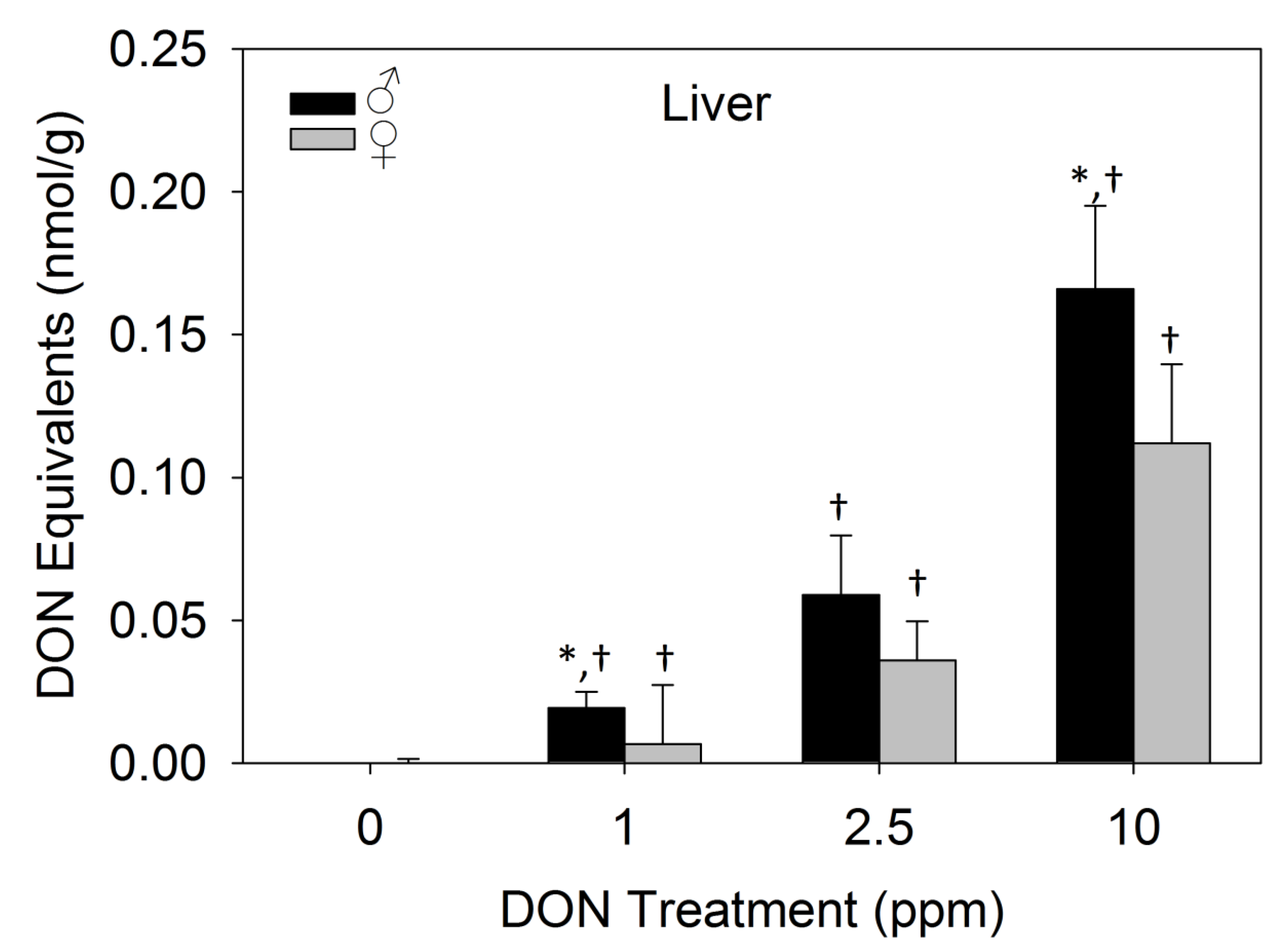
2.3.4. Proinflammatory Plasma Cytokines Were not Detectable after 17 days of Dietary DON Exposure
3. Experimental Section
3.1. Animals
3.2. DON
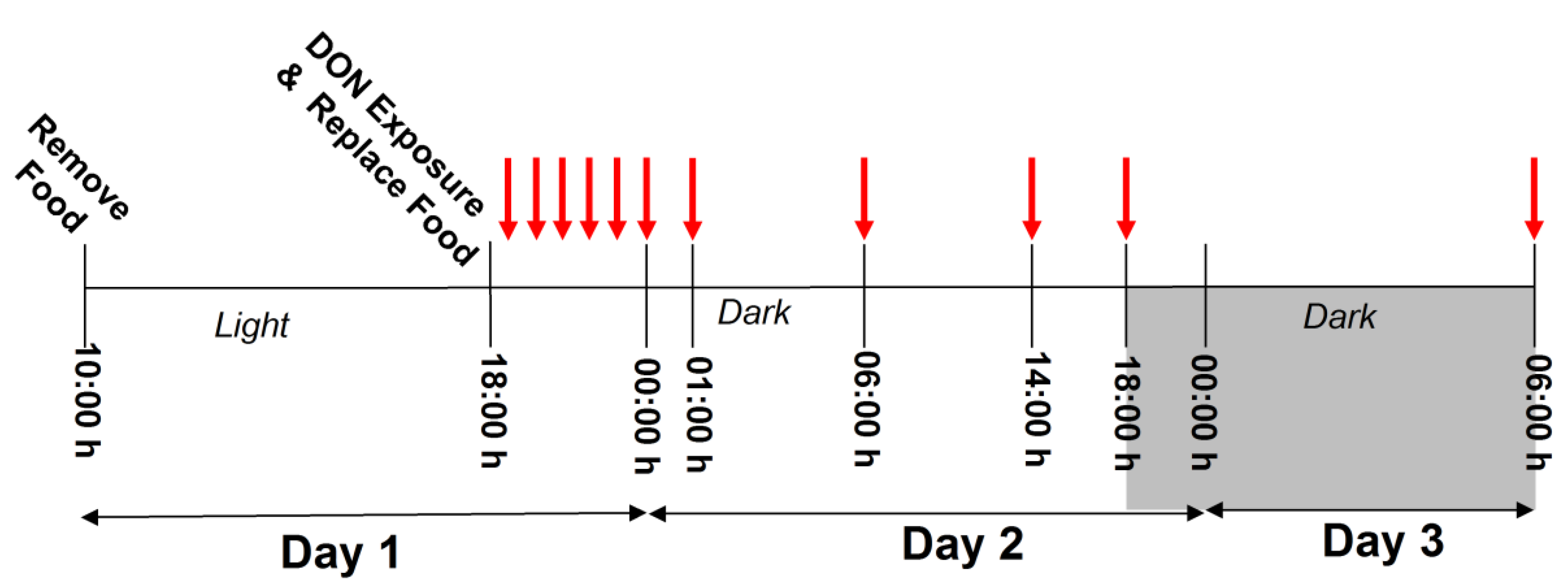
3.3. Experimental Design
3.3.1. Study 1
3.3.2. Study 2
3.3.3. Study 3
3.4. Analytical
3.4.1. DON Quantification
3.4.2. Proinflammatory Cytokine Analyses
3.4.3. Satiety Hormone Analyses
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, H.L.; Hamilton, R.M.G.; Friend, D.W.; Thompson, B.K.; Hartin, K.E. Feeding trials with vomitoxin (deoxynivalenol)-contaminated wheat—effects on swine, poultry, and dairy-cattle. J. Am. Vet. Med. Assoc. 1984, 185, 527–531. [Google Scholar] [PubMed]
- Iverson, F.; Armstrong, C.; Nera, E.; Truelove, J.; Fernie, S.; Scott, P.; Stapley, R.; Hayward, S.; Gunner, S. Chronic feeding study of deoxynivalenol in B6C3F1 male and female mice. Teratog. Carcinog. Mutagen. 1995, 15, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Amuzie, C.J.; Pestka, J.J. Suppression of insulin-like growth factor acid-labile subunit expression-a novel mechanism for deoxynivalenol-induced growth retardation. Toxicol. Sci. 2010, 113, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A. A new perspective on deoxynivalenol and growth suppression. Toxicol. Sci. 2010, 113, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Bates, M.A.; Bursian, S.J.; Link, J.E.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.B.; Pestka, J.J. Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon x, and nivalenol. Toxicol. Sci. 2013, 131, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, D.M.; Yoshizawa, T.; Morooka, N.; Tuite, J. Emetic and refusal activity of deoxynivalenol to swine. Appl. Environ. Microbiol. 1977, 34, 547–552. [Google Scholar] [PubMed]
- Forsell, J.H.; Witt, M.F.; Tai, J.H.; Jensen, R.; Pestka, J.J. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem. Toxicol. 1986, 24, 213–219. [Google Scholar] [CrossRef]
- Flannery, B.M.; Wu, W.D.; Pestka, J.J. Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem. Toxicol. 2011, 49, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.M.; Bondy, G.S.; Azconaolivera, J.I.; Pestka, J.J. Role of gender and strain in vomitoxin-induced dsyregulation of iga production and iga nephropathy in the mouse. J. Toxicol. Environ. Health 1994, 43, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.M.; Beasley, V.R.; Bratich, P.M.; Swanson, S.P.; Shivaprasad, H.L.; Buck, W.B. Sex-related reduced weight gains in growing swine fed diets containing deoxynivalenol. J. Anim. Sci. 1985, 61, 942–950. [Google Scholar] [PubMed]
- Rotter, B.A.; Thompson, B.K.; Rotter, R.G. Optimization of the mouse bioassay for deoxynivalenol as an alternative to large animal studies. Bull. Environ. Contam. Toxicol. 1994, 53, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.M.; Azcona-Olivera, J.I.; Pestka, J.J. Vomitoxin (deoxynivalenol)-induced iga nephropathy in the B6C3F1 mouse - dose-response and male predilection. Toxicology 1994, 92, 245–260. [Google Scholar] [CrossRef]
- Andretta, I.; Kipper, M.; Lehnen, C.R.; Hauschild, L.; Vale, M.M.; Lovatto, P.A. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal 2012, 6, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.M.; Azcona-Olivera, J.I.; Murtha, J.M.; Pestka, J.J. Effects of dihydrotestosterone and estradiol on experimental IgA nephropathy induced by vomitoxin. Fundam. Appl. Toxicol. 1995, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine-induced sickness behavior: Mechanisms and implications. In Role of Neural Plasticity in Chemical Intolerance; Sorg, B.A., Bell, I.R., Eds.; 2001; Volume 933, pp. 222–234. [Google Scholar]
- Lebrun, B.; Tardivel, C.; Félix, B.; Abysique, A.; Troadec, J.-D.; Gaigé, S.; Dallaporta, M. Dysregulation of energy balance by trichothecene mycotoxins: Mechanisms and prospects. Neurotoxicology 2015, 49, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Mounien, L.; Trouslard, J.; et al. Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: A PGE2-independent mechanism. Toxicol. Sci. 2011, 124, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Azcona-Olivera, J.I.; Ouyang, Y.; Murtha, J.; Chu, F.S.; Pestka, J.J. Induction of cytokine messenger-RNAs in mice after oral-exposure to the trichothecene vomitoxin (deoxynivalenol)—relationship to toxin distribution and protein-synthesis inhibition. Toxicol. Appl. Pharmacol. 1995, 133, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Amuzie, C.J. Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: Comparison of weanling and adult mice. Food Chem. Toxicol. 2008, 46, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.M.; Clark, E.S.; Pestka, J.J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide yy. Toxicol. Sci. 2012, 130, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Zhou, H.R.; He, K.Y.; Pan, X.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.B.; Pestka, J.J. Role of cholecystokinin in anorexia induction following oral exposure to the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon x, and nivalenol. Toxicol. Sci. 2014, 138, 278–289. [Google Scholar] [CrossRef] [PubMed]
- De Lartigue, G.; Dimaline, R.; Varro, A.; Dockray, G.J. Cocaine- and amphetamine-regulated transcript: Stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J. Neurosci. 2007, 27, 2876–2882. [Google Scholar] [CrossRef] [PubMed]
- Challis, B.G.; Pinnock, S.B.; Coll, A.P.; Carter, R.N.; Dickson, S.L.; O’Rahilly, S. Acute effects of PYY3–36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem. Biophys. Res. Commun. 2003, 311, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Islam, Z.; Amuzie, C.J. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 2008, 178, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Trenholm, H.L. Tissue distribution of deoxynivalenol in swine dosed intravenously. J. Agric. Food Chem. 1991, 39, 748–751. [Google Scholar] [CrossRef]
- Buckley, D.B.; Klaassen, C.D. Tissue- and gender-specific mrna expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab. Dispos. 2007, 35, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.B.; Klaassen, C.D. Mechanism of gender-divergent UDP-glucuronosyltransferase mrna expression in mouse liver and kidney. Drug Metab. Dispos. 2009, 37, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Amuzie, C.J.; Shinozuka, J.; Pestka, J.J. Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol. Sci. 2009, 111, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Pestka, J.J. Role of IL-1 beta in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice. Toxicol. Sci. 2003, 74, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Jeong, S.-H.; Cho, J.-H.; Shin, H.-S.; Son, S.-W.; Yeo, Y.-K.; Kang, H.-G. Effects of oral deoxynivalenol exposure on immune-related parameters in lymphoid organs and serum of mice vaccinated with porcine parvovirus vaccine. Mycotoxin Res. 2013, 29, 185–192. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, E.S.; Flannery, B.M.; Pestka, J.J. Murine Anorectic Response to Deoxynivalenol (Vomitoxin) Is Sex-Dependent. Toxins 2015, 7, 2845-2859. https://doi.org/10.3390/toxins7082845
Clark ES, Flannery BM, Pestka JJ. Murine Anorectic Response to Deoxynivalenol (Vomitoxin) Is Sex-Dependent. Toxins. 2015; 7(8):2845-2859. https://doi.org/10.3390/toxins7082845
Chicago/Turabian StyleClark, Erica S., Brenna M. Flannery, and James J. Pestka. 2015. "Murine Anorectic Response to Deoxynivalenol (Vomitoxin) Is Sex-Dependent" Toxins 7, no. 8: 2845-2859. https://doi.org/10.3390/toxins7082845





