Deoxynivalenol & Deoxynivalenol-3-Glucoside Mitigation through Bakery Production Strategies: Effective Experimental Design within Industrial Rusk-Making Technology
Abstract
:1. Introduction
2. Results and Discussion
| Treatment | Rusk Making | ||
|---|---|---|---|
| Minimum | Optimum | Maximum | |
| DON bran level (μg/kg) | 600 | 1050 | 1500 |
| Dextrose (%) | 2 | 4 | 6 |
| Yeast (%) | 2 | 3 | 4 |
| Promoting agents (%) | 0 | 0.5 | 1 |
| Fermentation time (min) | 40 | 50 | 60 |
| Fermentation temperature (°C) | 26 | 36 | 46 |
| Baking time (min) | 12 | 21 | 30 |
| Baking temperature (°C) | 180 | 195 | 210 |
| Toasting time (min) | 15 | 20 | 25 |
| Toasting temperature (°C) | 110 | 130 | 150 |
| Experiment Number | DON in Bran (μg/kg d.m.) 1 | Dextrose (%) | Yeast (%) | Promoting Agent (%) | Fermentation Stage | Baking Stage | Toasting Stage | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Temperature (°C) | Time (min) | Temperature (°C) | Time (min) | Temperature (°C) | |||||
| 1 | 600 ± 16 | 2 | 2 | 0 | 40 | 26 | 12 | 180 | 25 | 150 |
| 2 | 1500 ± 92 | 2 | 2 | 0 | 60 | 26 | 30 | 210 | 15 | 110 |
| 3 | 600 ± 16 | 6 | 2 | 0 | 60 | 46 | 12 | 210 | 15 | 110 |
| 4 | 1500 ± 92 | 6 | 2 | 0 | 40 | 46 | 30 | 180 | 25 | 150 |
| 5 | 600 ± 16 | 2 | 4 | 0 | 60 | 46 | 30 | 180 | 15 | 150 |
| 6 | 1500 ± 92 | 2 | 4 | 0 | 40 | 46 | 12 | 210 | 25 | 110 |
| 7 | 600 ± 16 | 6 | 4 | 0 | 40 | 26 | 30 | 210 | 25 | 110 |
| 8 | 1500 ± 92 | 6 | 4 | 0 | 60 | 26 | 12 | 180 | 15 | 150 |
| 9 | 600 ± 16 | 2 | 2 | 1 | 40 | 46 | 30 | 210 | 15 | 150 |
| 10 | 1500 ± 92 | 2 | 2 | 1 | 60 | 46 | 12 | 180 | 25 | 110 |
| 11 | 600 ± 16 | 6 | 2 | 1 | 60 | 26 | 30 | 180 | 25 | 110 |
| 12 | 1500 ± 92 | 6 | 2 | 1 | 40 | 26 | 12 | 210 | 15 | 150 |
| 13 | 600 ± 16 | 2 | 4 | 1 | 60 | 26 | 12 | 210 | 25 | 150 |
| 14 | 1500 ± 92 | 2 | 4 | 1 | 40 | 26 | 30 | 180 | 15 | 110 |
| 15 | 600 ± 16 | 6 | 4 | 1 | 40 | 46 | 12 | 180 | 15 | 110 |
| 16 | 1500 ± 92 | 6 | 4 | 1 | 60 | 46 | 30 | 210 | 25 | 150 |
| 17 | 1050 ± 48 | 4 | 3 | 0.5 | 50 | 36 | 21 | 195 | 20 | 130 |
| 18 | 1050 ± 48 | 4 | 3 | 0.5 | 50 | 36 | 21 | 195 | 20 | 130 |
| 19 | 1050 ± 48 | 4 | 3 | 0.5 | 50 | 36 | 21 | 195 | 20 | 130 |
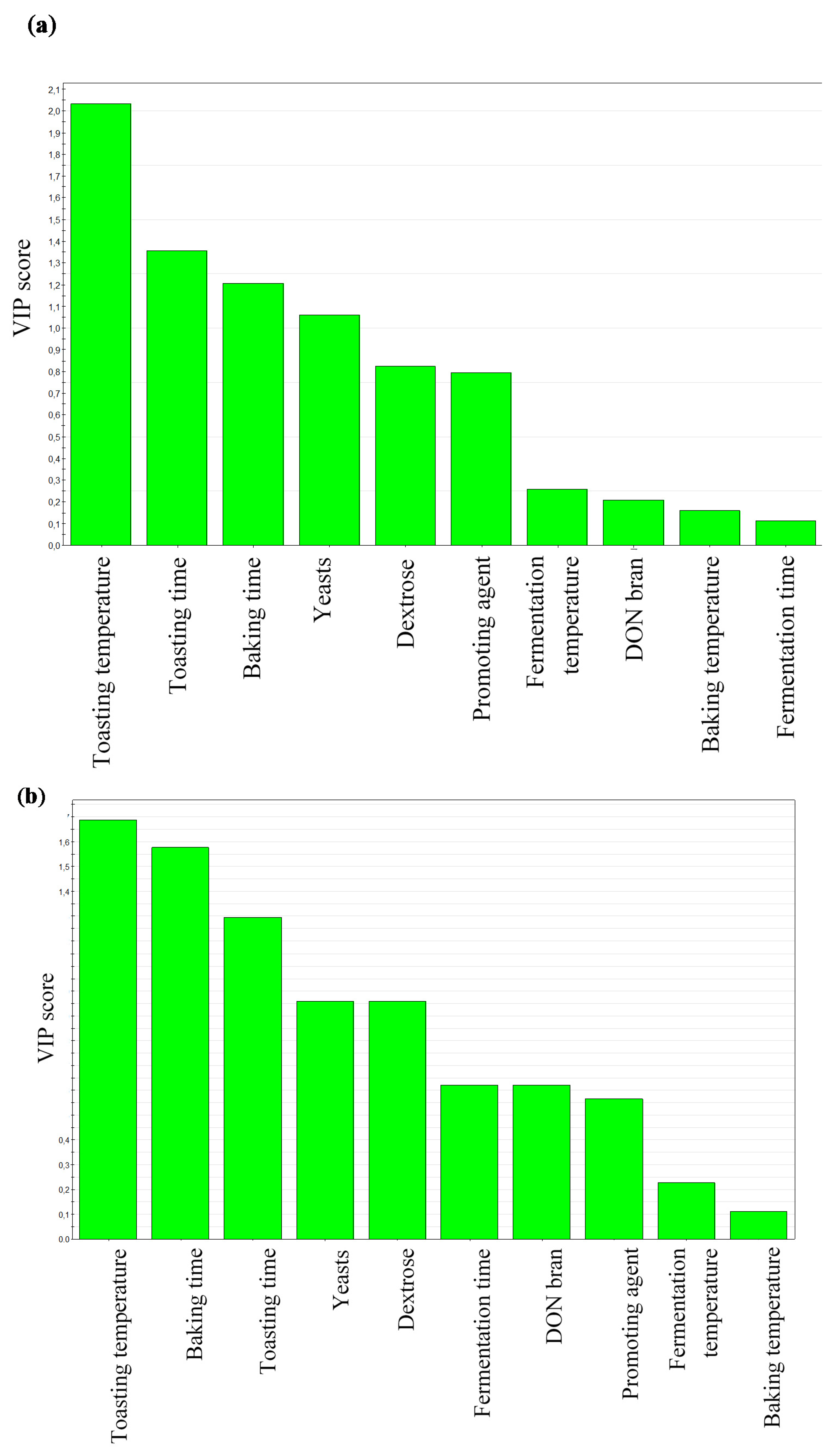
DON and DON3Glc Levels during Rusk-Making
| Experiment Number | DON in Bran (μg/kg d.m.) 1 | MC 2 (%) | DON in Dough (μg/kg d.m.) 1 | MC 2 (%) | DON in Fermented Dough (μg/kg d.m.) 1 | MC 2 (%) | DON in Baked Rusk (μg/kg d.m.) 1 | MC 2 (%) | DON in Toasted Rusk (μg/kg d.m.) 1 | MC 2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 600 ± 16 | 12 | 273 ± 25 | 43 | 273 ± 29 | 43 | 239 ± 10 | 38 | 194 ± 16 | 3 |
| 2 | 1500 ± 92 | 12 | 349 ± 34 | 41 | 340 ± 25 | 40 | 334 ± 12 | 33 | 329 ± 7 | 10 |
| 3 | 600 ± 16 | 12 | 379 ± 21 | 42 | 333 ± 11 | 39 | 338 ± 5 | 36 | 322 ± 20 | 14 |
| 4 | 1500 ± 92 | 12 | 348 ± 30 | 41 | 359 ± 18 | 41 | 351 ± 23 | 33 | 199 ± 16 | 1 |
| 5 | 600 ± 16 | 12 | 259 ± 15 | 43 | 261 ± 12 | 42 | 246 ± 12 | 32 | 188 ± 20 | 5 |
| 6 | 1500 ± 92 | 12 | 392 ± 30 | 41 | 339 ± 26 | 41 | 360 ± 15 | 37 | 317 ± 11 | 5 |
| 7 | 600 ± 16 | 12 | 257 ± 16 | 44 | 242 ± 10 | 44 | 199 ± 24 | 33 | 172 ± 28 | 4 |
| 8 | 1500 ± 92 | 12 | 306 ± 12 | 41 | 300 ± 5 | 41 | 324 ± 15 | 37 | 239 ± 15 | 5 |
| 9 | 600 ± 16 | 12 | 242 ± 18 | 44 | 210 ± 22 | 42 | 198 ± 15 | 33 | 165 ± 26 | 4 |
| 10 | 1500 ± 92 | 12 | 339 ± 31 | 44 | 333 ± 15 | 44 | 343 ± 12 | 36 | 315 ± 10 | 6 |
| 11 | 600 ± 16 | 12 | 213 ± 14 | 39 | 220 ± 18 | 38 | 229 ± 19 | 38 | 200 ± 11 | 5 |
| 12 | 1500 ± 92 | 12 | 318 ± 27 | 43 | 298 ± 32 | 42 | 347 ± 36 | 38 | 290 ± 15 | 2 |
| 13 | 600 ± 16 | 12 | 253 ± 12 | 40 | 256 ± 20 | 40 | 243 ± 20 | 38 | 149 ± 19 | 6 |
| 14 | 1500 ± 92 | 12 | 339 ± 31 | 39 | 311 ± 23 | 38 | 330 ± 18 | 37 | 308 ± 12 | 4 |
| 15 | 600 ± 16 | 12 | 211 ± 18 | 40 | 215 ± 26 | 40 | 253 ± 3 | 38 | 212 ± 5 | 6 |
| 16 | 1500 ± 92 | 12 | 280 ± 14 | 41 | 313 ± 21 | 43 | 197 ± 32 | 29 | 68 ± 11 | 1 |
| 17 | 1050 ± 48 | 11 | 219 ± 10 | 45 | 229 ± 19 | 44 | 225 ± 21 | 38 | 194 ± 16 | 5 |
| 18 | 1050 ± 48 | 11 | 272 ± 8 | 45 | 248 ± 30 | 44 | 225 ± 19 | 38 | 196 ± 12 | 5 |
| 19 | 1050 ± 48 | 11 | 251 ± 20 | 45 | 213 ± 19 | 44 | 218 ± 24 | 38 | 186 ± 21 | 5 |
| Experiment Number | D3Glc Dough (μg/kgd.m.) 1 | MC 2 (%) | D3Glc Fermented Dough (μg/kg d.m.) 1 | MC 2 (%) | D3Glc Baked Rusk (μg/kg d.m.) 1 | MC 2 (%) | D3Glc Toasted Rusk (μg/kg d.m.) 1 | MC 2 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 ± 6 | 43 | 29 ± 5 | 43 | 22 ± 6 | 38 | 11 ± 5 | 3 |
| 2 | 77 ± 10 | 41 | 77 ± 9 | 40 | 27 ± 5 | 33 | 23 ± 3 | 10 |
| 3 | 36 ± 3 | 42 | 41 ± 4 | 39 | 30 ± 3 | 36 | 25 ± 4 | 14 |
| 4 | 46 ± 7 | 41 | 64 ± 9 | 41 | 31 ± 4 | 33 | 6 ± 1 | 1 |
| 5 | 23 ± 4 | 43 | 22 ± 5 | 42 | 18 ± 2 | 32 | 12 ± 3 | 5 |
| 6 | 60 ± 6 | 41 | 64 ± 7 | 41 | 34 ± 5 | 37 | 21 ± 4 | 5 |
| 7 | 18 ± 3 | 44 | 18 ± 4 | 44 | 8 ± 1 | 33 | 6 ± 2 | 4 |
| 8 | 39 ± 4 | 41 | 45 ± 6 | 41 | 23 ± 3 | 37 | 18 ± 3 | 5 |
| 9 | 23 ± 2 | 44 | 29 ± 3 | 42 | 12 ± 2 | 33 | 7 ± 0 | 4 |
| 10 | 54 ± 5 | 44 | 65 ± 7 | 44 | 32 ± 4 | 36 | 27 ± 6 | 6 |
| 11 | 19 ± 3 | 39 | 17 ± 2 | 38 | 14 ± 3 | 38 | 10 ± 2 | 5 |
| 12 | 41 ± 5 | 43 | 43 ± 3 | 42 | 30 ± 4 | 38 | 20 ± 5 | 2 |
| 13 | 26 ± 2 | 40 | 29 ± 2 | 40 | 19 ± 3 | 38 | 8 ± 4 | 6 |
| 14 | 41 ± 2 | 39 | 61 ± 5 | 38 | 28 ± 3 | 37 | 20 ± 5 | 4 |
| 15 | 17 ± 3 | 40 | 19 ± 2 | 40 | 14 ± 2 | 38 | 10 ± 3 | 6 |
| 16 | 30 ± 4 | 41 | 40 ± 3 | 43 | 9 ± 1 | 29 | <0.1 | 1 |
| 17 | 17 ± 3 | 45 | 30 ± 5 | 44 | 16 ± 4 | 38 | 9 ± 1 | 5 |
| 18 | 11 ± 5 | 45 | 19 ± 2 | 44 | 15 ± 3 | 38 | 8 ± 2 | 5 |
| 19 | 17 ± 3 | 45 | 24 ± 4 | 44 | 14 ± 5 | 38 | 9 ± 1 | 5 |
3. Experimental Section
3.1. Chemicals
3.2. Raw Material Selection and Preparation
3.3. Moisture Content Determination
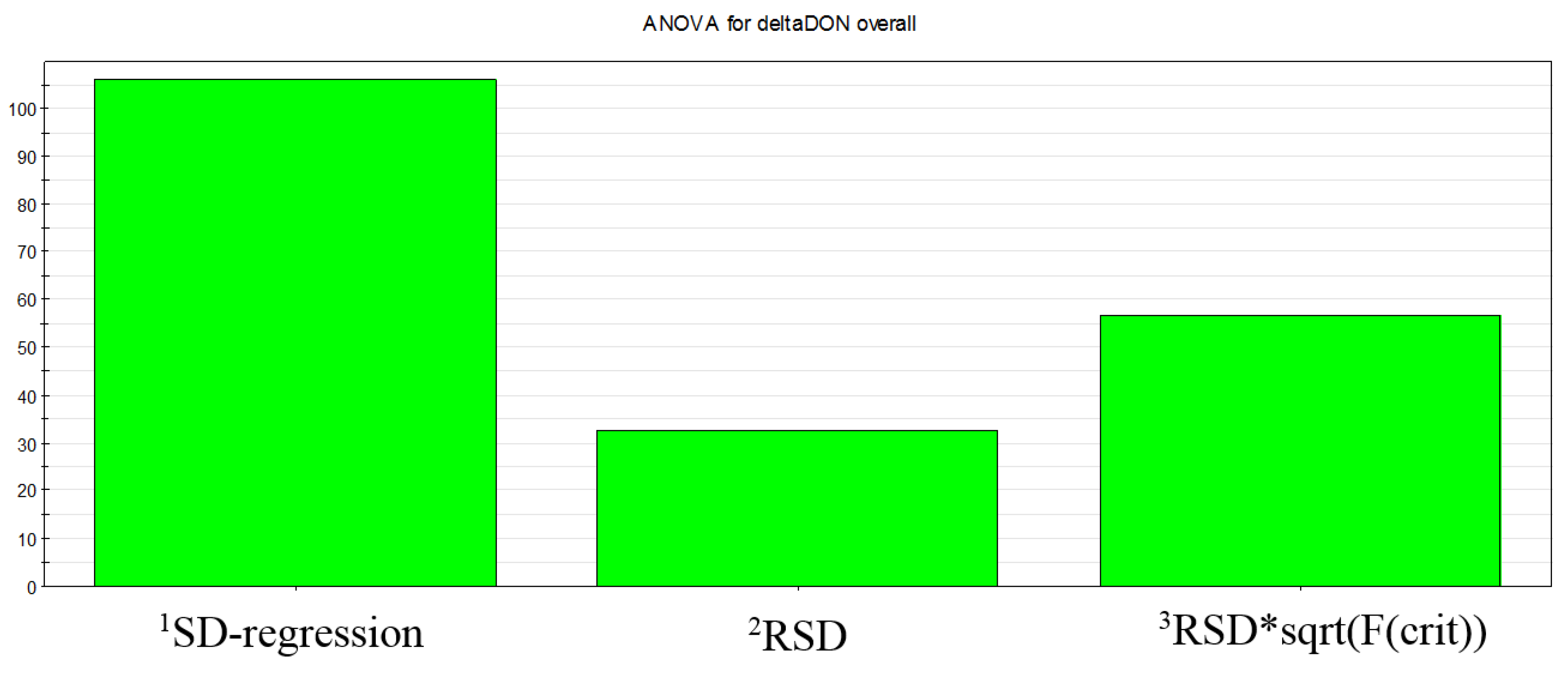
3.4. Experimental Design
3.5. Dough Preparation and Rusk-Making
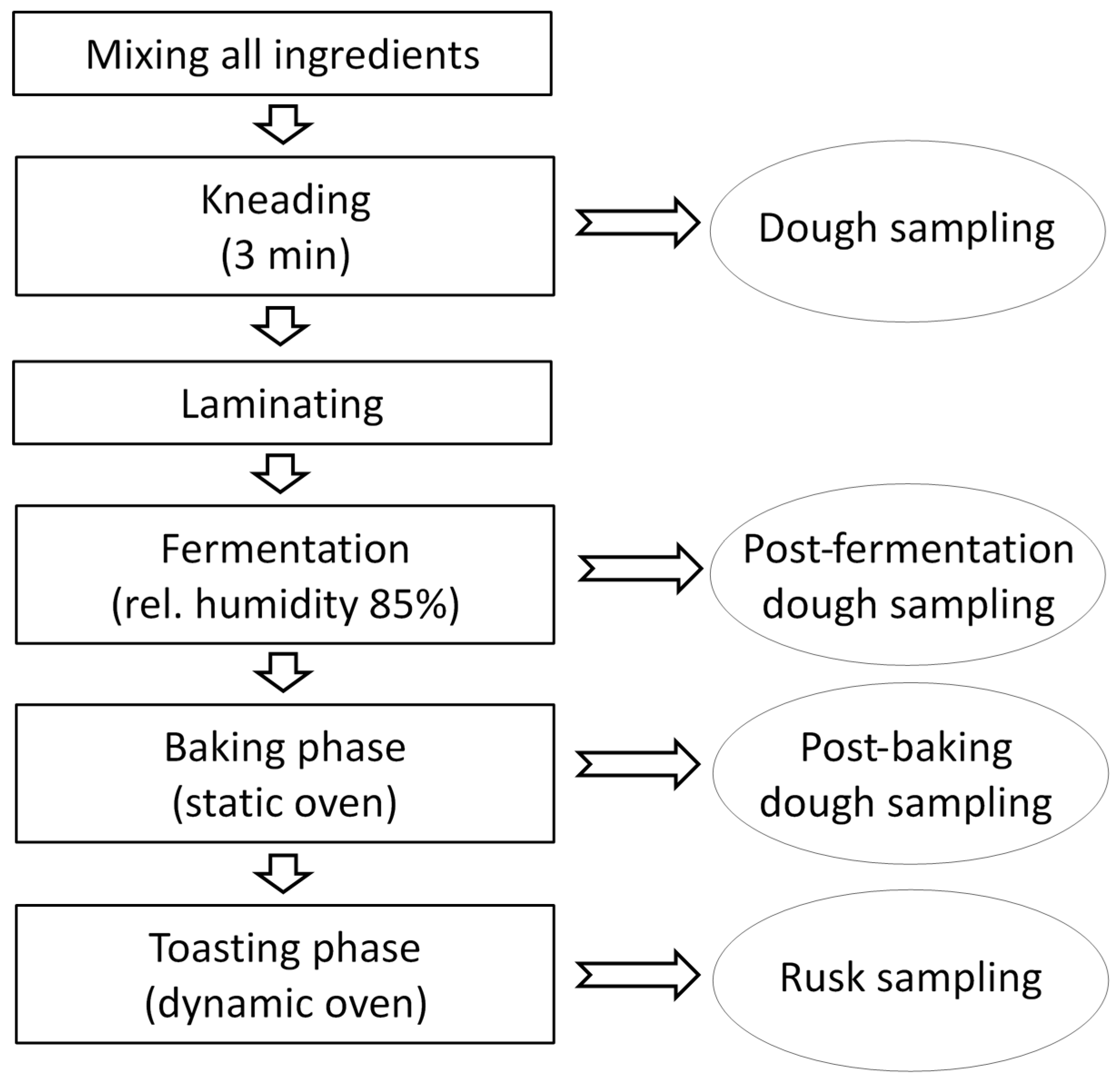
3.6. Sample Extraction
3.7. Instrumental Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dimitri, C.; Oberholzer, L. EU and US organic markets face strong demand under different policies. Amber Waves 2006, 4, 12–19. [Google Scholar]
- Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT). Available online: http://faostat.fao.org (accessed on 23 February 2015).
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Ritieni, A.; Piva, G.; Pietri, A. Mycotoxins in the Human Food Chain. In The Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 187–224. [Google Scholar]
- Larsen, J.C.; Hunt, J.; Perrin, I.; Ruckenbauer, P. Workshop on trichothecenes with a focus on DON: summary report. Toxicol. Lett. 2004, 153, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Pascale, M. An overview on Fusarium mycotoxins in the durum wheat pasta production chain. Cereal Chem. 2010, 87, 21–27. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation (EC) No. 165/2010 of 26/2/2010, setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2010, L446, 1–127. [Google Scholar]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T. Risk Assessment and Risk Management of Mycotoxins in Food. In Mycotoxins in Food, Detection and Control; Magan, N., Olsen, M., Eds.; Woodhead Publishing: Cambridge, UK, 2004; Volume 6, pp. 3–31. [Google Scholar]
- Suman, M.; Generotti, S. Transformation of Mycotoxins upon Food Processing: Masking, Binding and Degradation Phenomena. In Masked Mycotoxins in Food: Formation, Occurrence and Toxicological Relevance; RSC Publishing: London, UK, 2015; in press. [Google Scholar]
- Scudamore, K.A. Fate of Fusarium mycotoxins in the cereal industry: Recent UK studies. World Mycotox. J. 2008, 1, 315–325. [Google Scholar] [CrossRef]
- Brera, C.; Catano, C.; de Santis, B.; de Begnach, F.; de Giacomo, M.; Pannunzi, E.; Miraglia, M. Effect of industrial processing on the distribution of aflatoxins and zearalenone in corn milling fractions. J. Agric. Food Chem. 2006, 54, 5014–5019. [Google Scholar] [CrossRef] [PubMed]
- Lancova, K.; Hajslova, J.; Kostelanska, M.; Kohoutkova, J.; Nedelnik, J.; Moravcova, H.; Vanova, M. Fate of trichothecene mycotoxins during the processing: Milling and baking. Food Addit. Contam. 2008, 25, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Tibola, C.S.; Fernandes, J.M.C.; Guarienti, E.M.; Nicolau, M. Distribution of Fusarium mycotoxins in wheat milling process. Food Control 2015, 53, 91–95. [Google Scholar] [CrossRef]
- Samar, M.M.; Resnik, S.L.; Gonzalez, H.H.L.; Pacin, A.M.; Castillo, M.D. Deoxynivalenol reduction during the frying process of turnover pie covers. Food Control 2007, 18, 1295–1299. [Google Scholar] [CrossRef]
- Voss, K.A.; Snook, M.E. Stability of the mycotoxin deoxynivalenol (DON) during the production of flour-based foods and wheat flake cereal. Food Add. Contam. 2010, 27, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Sanchis, V.; Ramos, A.J.; Marίn, S. Thermal stability and kinetics of degradation of deoxynivalenol, deoxynivalenol conjugates and ochratoxin A during baking of wheat bakery products. Food Chem. 2015, 178, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, B. Evaluation on levels and conversion profiles of DON, 3-ADON, and 15-ADON during bread making process. Food Chem. 2015, 185, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, B.H.; Munich, M.; Kleijer, G.; Mascher, F. Characterization of kernel resistance against Fusarium infection in spring wheat by baking quality and mycotoxin assessments. Eur. J. Plant. Pathol. 2008, 120, 61–68. [Google Scholar] [CrossRef]
- Numanoglu, E.; Uygun, U.; Koksel, H.; Solfrizzo, M. Stability of Fusarium toxins during traditional Turkish maize bread production. Qual. Assur. Saf. Crop. 2010, 2, 84–92. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B. Fates of deoxynivalenol and deoxynivalenol-3-glucoside during bread and noodle processing. Food Control 2015, 50, 754–757. [Google Scholar] [CrossRef]
- Pacin, A.; Bovier, E.C.; Cano, G.; Taglieri, D.; Pezzani, C.H. Effect of the bread making process on wheat flour contaminated by deoxynivalenol and exposure estimate. Food Control 2010, 21, 1004–1010. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Hazel, C.M.; Patel, S.; Scriven, F. Deoxynivalenol and other Fusarium mycotoxins in bread, cake, and biscuits produced from UK-grown wheat under commercial and pilot scale conditions. Food Addit. Contam. 2009, 26, 1191–1198. [Google Scholar] [CrossRef]
- Valle-Algarra, F.M.; Mateo, E.M.; Medina, A.; Gimeno-Adelantado, J.V.; Jimenéz, M. Changes in ochratoxin A and type A and type B trichothecenes contained in wheat flour during dough fermentation and bread making. Food Addit. Contam. 2009, 26, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, E.; Catellani, D.; Dall’Asta, C.; Galaverna, G.; Dossena, A.; Marchelli, R.; Suman, M. Fate of Fusarium mycotoxins in the cereal product supply chain: The deoxynivalenol (DON) case within industrial bread-making technology. Food Addit. Contam. 2010, 27, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Marìn, S.; Morales, H.; Ramos, A.J.; Sanchis, V. The fate of deoxynivalenol and ochratoxin A during the breadmaking process, effects of sourdough use and bran content. Food Chem. Toxicol. 2014, 68, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; de Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by an UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Morales, H.; Sanchis, V.; Ramos, A.J.; Marìn, S. Stability of DON and OTA during the breadmaking process and determination of process and performance criteria. Food Control 2013, 40, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Zachariasova, M.; Vaclavikova, M.; Lacina, O.; Vaclavik, L.; Hajslova, J. Deoxynivalenol oligoglycosides: New “masked” Fusarium toxins occurring in malt, beer, and breadstuff. J. Agric. Food Chem. 2012, 60, 9280–9291. [Google Scholar] [CrossRef] [PubMed]
- Kostelanska, M.; Dzuman, Z.; Malachova, A.; Capouchova, I.; Prokinova, E.; Skerikova, A.; Hajslova, J. Effects of milling and baking technologies on levels of deoxynivalenol and its masked form deoxynivalenol-3-glucoside. J. Agric. Food Chem. 2011, 59, 9303–9312. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Burgess, K.; Whitney, K.L.; Gu, Y.; Qian, S.Y. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in wheat. Food Control 2012, 26, 287–292. [Google Scholar] [CrossRef]
- European Commission (EC). Proposal for a Regulation of the European Parliament and of the Council on Animal Health; European Commission (EC): Brussels, Belgium, 2013. [Google Scholar]
- European Food Safety Authority (EFSA). Request for a Scientific Opinion on the Risk for Animal and Public Health Related to the Presence of Deoxynivalenol, Metabolites of Deoxynivalenol and Masked Deoxynivalenol in Food and Feed; European Food Safety Authority (EFSA): Parma, Italy, 2013. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar]
- European Commission (EC). Commission Recommendation 2013/165/EU of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- Suman, M.; Manzitti, A.; Catellani, D. A Design of Experiments approach to studying Deoxynivalenol and Deoxynivalenol-3-glucoside evolution throughout industrial production of wholegrain crackers exploiting LC-MS/MS techniques. World Mycotox. J. 2012, 5, 241–249. [Google Scholar] [CrossRef]
- Suman, M.; Bergamini, E.; Catellani, D.; Manzitti, A. Development and validation of a liquid chromatography/linear ion trap mass spectrometry method for the quantitative determination of deoxynivalenol-3-glucoside in processed cereal-derived products. Food Chem. 2013, 136, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Telford, J.K. A brief introduction to design of experiments. John Hopkins APL Technical Dig. 2007, 27, 224–232. [Google Scholar]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union 2002, L221, 8–36. [Google Scholar]
- Malachova, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generotti, S.; Cirlini, M.; Malachova, A.; Sulyok, M.; Berthiller, F.; Dall'Asta, C.; Suman, M. Deoxynivalenol & Deoxynivalenol-3-Glucoside Mitigation through Bakery Production Strategies: Effective Experimental Design within Industrial Rusk-Making Technology. Toxins 2015, 7, 2773-2790. https://doi.org/10.3390/toxins7082773
Generotti S, Cirlini M, Malachova A, Sulyok M, Berthiller F, Dall'Asta C, Suman M. Deoxynivalenol & Deoxynivalenol-3-Glucoside Mitigation through Bakery Production Strategies: Effective Experimental Design within Industrial Rusk-Making Technology. Toxins. 2015; 7(8):2773-2790. https://doi.org/10.3390/toxins7082773
Chicago/Turabian StyleGenerotti, Silvia, Martina Cirlini, Alexandra Malachova, Michael Sulyok, Franz Berthiller, Chiara Dall'Asta, and Michele Suman. 2015. "Deoxynivalenol & Deoxynivalenol-3-Glucoside Mitigation through Bakery Production Strategies: Effective Experimental Design within Industrial Rusk-Making Technology" Toxins 7, no. 8: 2773-2790. https://doi.org/10.3390/toxins7082773






