Extracts of Renealmia alpinia (Rottb.) MAAS Protect against Lethality and Systemic Hemorrhage Induced by Bothrops asper Venom: Insights from a Model with Extract Administration before Venom Injection
Abstract
:1. Introduction
2. Results
2.1. Inhibition of Lethal Activity
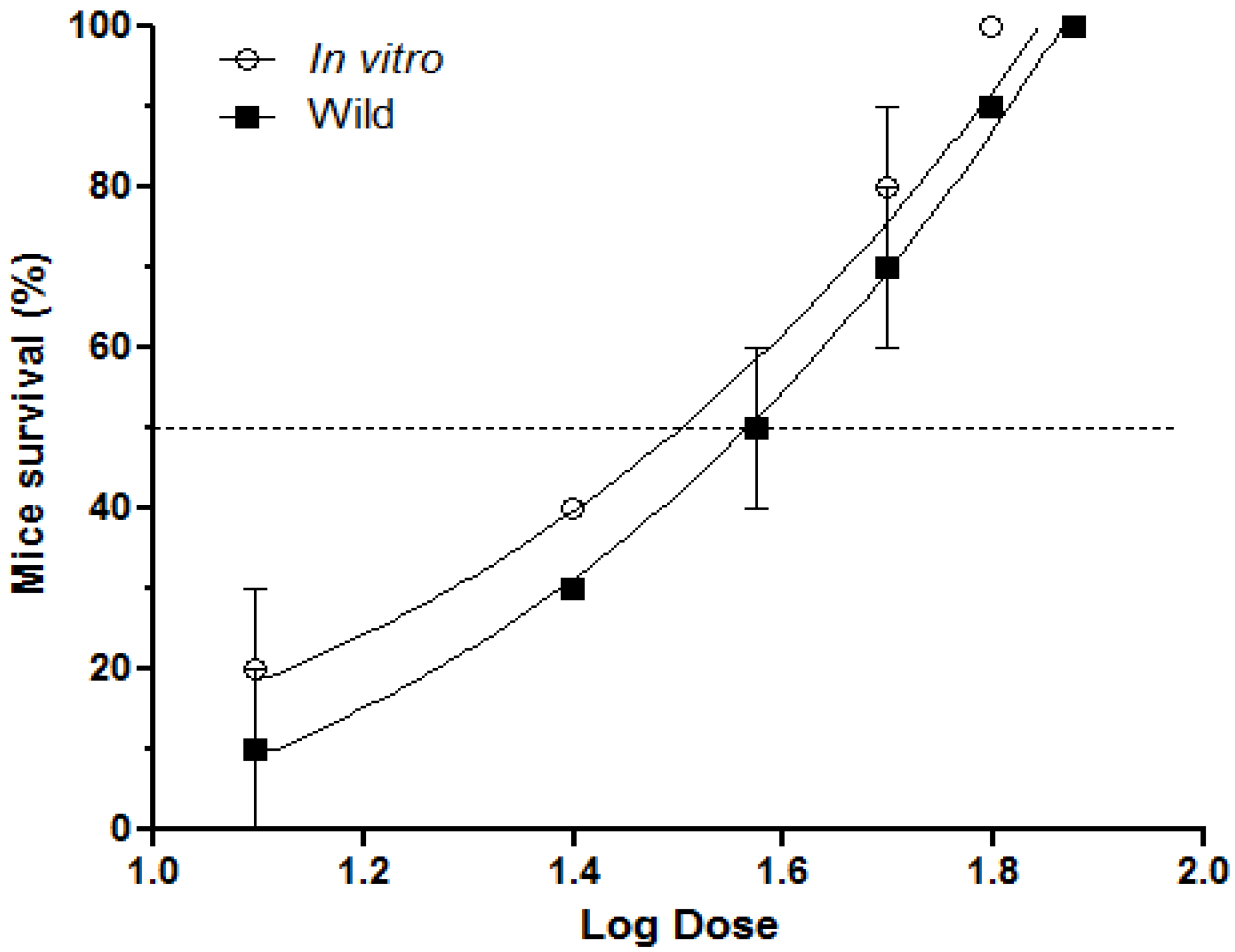
2.2. Inhibition of Pulmonary Hemorrhage
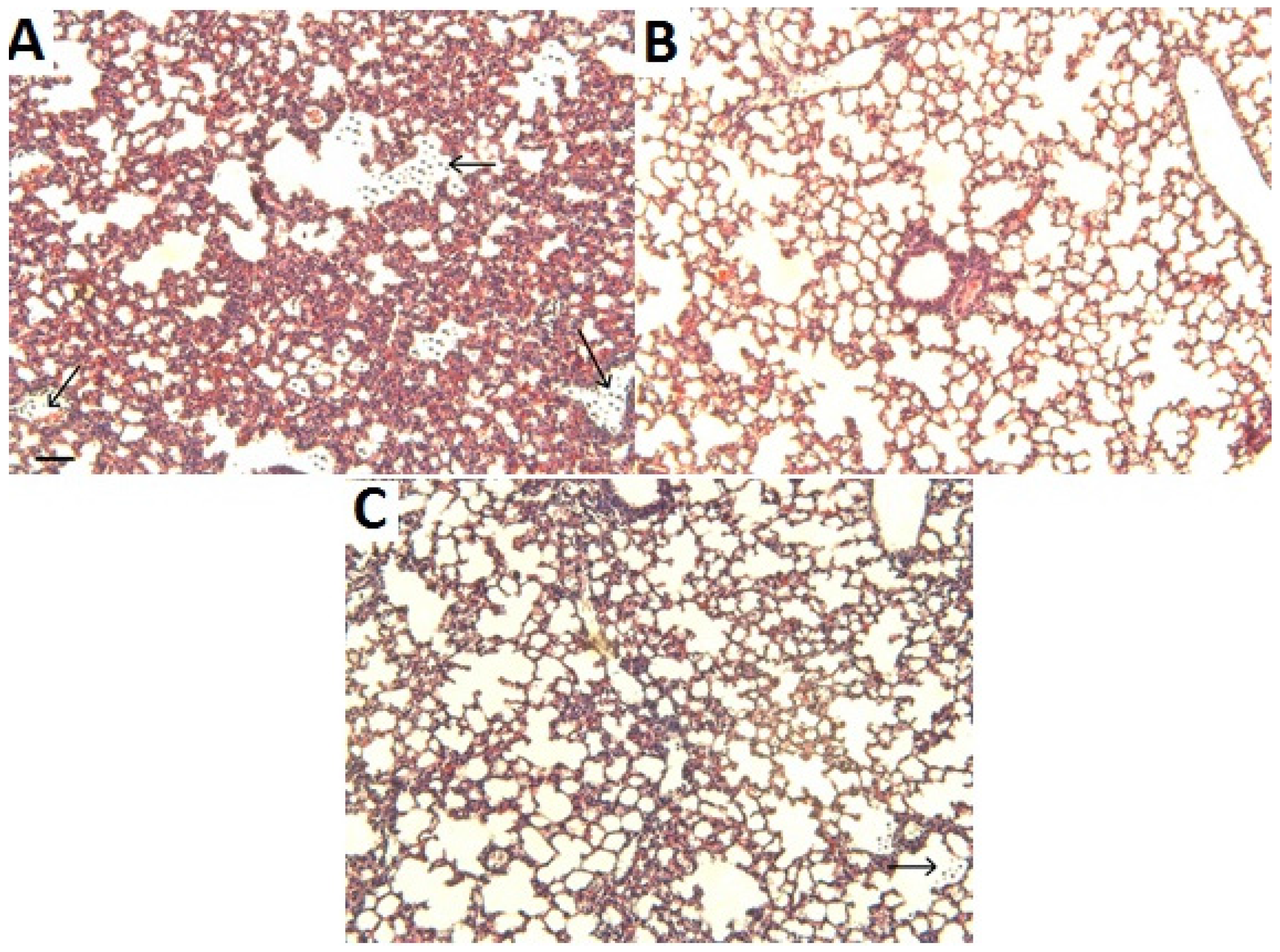
2.3. Histological Analysis
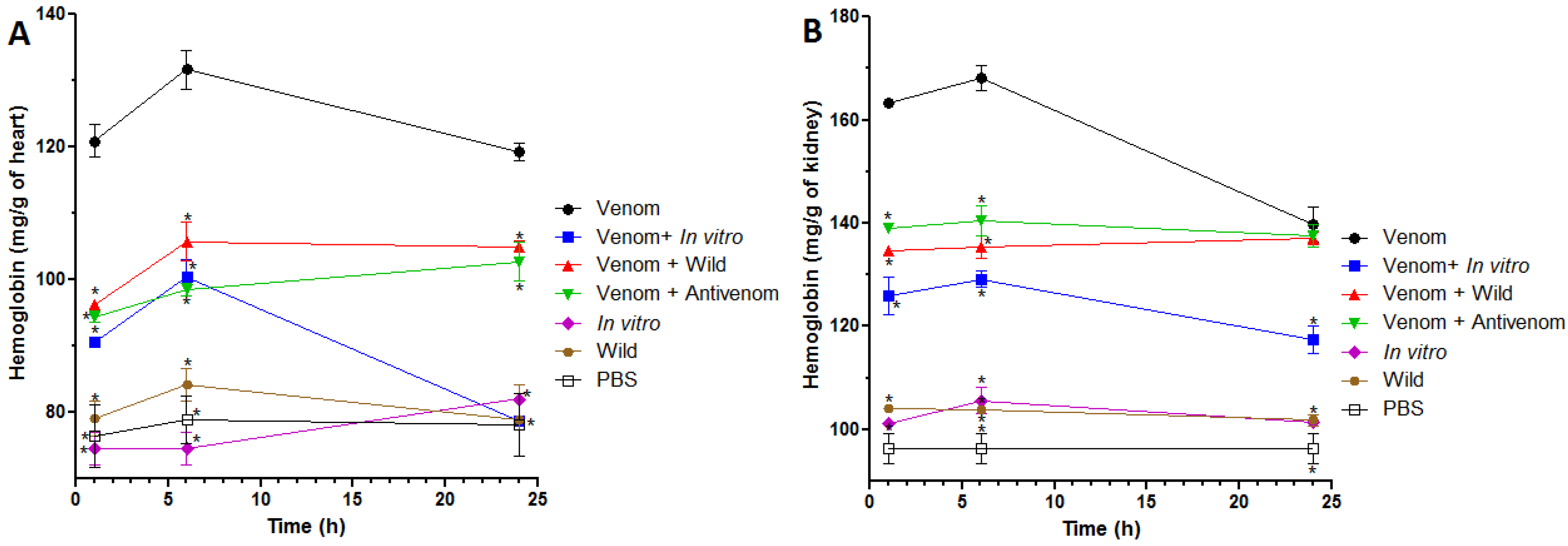
2.4. Inhibition of Venom-Induced Hemorrhage in Heart and Kidneys
3. Discussion
4. Experimental Section
4.1. Venom
4.2. Animals
4.3. Plant Material and Preparation of Extracts of R. alpinia
4.4. Inhibition of Lethal Activity of B. asper Venom by R. alpinia Extracts
4.5. Determination of the Minimum Pulmonary Hemorrhagic Dose
4.6. Inhibition of Venom-Induced Pulmonary Hemorrhage
4.7. Inhibition of Hemorrhage in Heart and Kidneys
4.8. Histological Studies
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gutiérrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 51, 80–92. [Google Scholar] [CrossRef]
- Warrell, D.A. Snakebites in Central and South America: Epidemiology, clinical features and clinical management. In The Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Comstock Publishing Associates: New York, NY, USA, 2004; pp. 709–761. [Google Scholar]
- Calvete, J.J. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev. Proteomics 2011, 8, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Otero-Patiño, R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A. Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon 2009, 54, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Bon, C. The serum—Therapie was discovered 100 years ago. Toxicon 1996, 34, 142–143. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- WHO. Rabies and Envenomings: A Neglected Public Health Issue: Report of a Consultative Meeting; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Soares, A.M.; Ticli, F.K.; Marcussi, S.; Lourenço, M.V.; Januário, A.H.; Sampaio, S.V.; Giglio, J.R.; Lomonte, B.; Pereira, P.S. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005, 12, 2625–2641. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, G. Plantas Comestibles en dos Comunidades de la Sierra Norte de Puebla: Xochitlán de Vicente Suárez y Zapotitlán de Méndez. Tesis de Licenciatura, Universidad Nacional Autónoma de México, México, D.F., Mexico, 1994; p. 315. [Google Scholar]
- Acero, L.E. Principales Plantas Útiles de la Amazonia Colombiana, Primera ed.; Editora Guadalupe: Bogotá, Colombia, 1979; p. 263. [Google Scholar]
- Standley, P.C.; Steyermark, J.A. Zingiberaceae. In Flora of Guatemala; Fieldiana: Botany Vol 24, part III; Chicago Natural History Museum: Chicago, IL, USA, 1952; p. 499. [Google Scholar]
- Otero, R.; Fonnegra, R.; Jiménez, S.L. Plantas Utilizadas Contra Mordeduras de Serpientes en Antioquia y Chocó, Colombia, Primera ed.; Granda-color: Medellín, Colombia, 2000. [Google Scholar]
- Núñez, V.; Otero, R.; Barona, J.; Saldarriaga, M.; Osorio, R.G.; Fonnegra, R. Neutralization of the edema-forming, defibrinating and coagulant effects of Bothrops asper venom by extracts of plants used by healers in Colombia. Braz. J. Med. Biol. Res. 2004, 37, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Fonnegra, R.; Jiménez, S.L.; Núñez, V.; Evans, N.; Alzate, S.A.; García, M.E.; Saldarriaga, M.; del Valle, G.; Osorio, R.G.; et al. Snakebites and ethnobotany in the northwest región of Colombia. Part I: Traditional use of plants. J. Ethnopharmacol. 2000, 71, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Núñez, V.; Barona, J.; Fonnegra, R.; Jiménez, S.L.; Osorio, R.G.; Saldarriaga, M.; Díaz, A. Snakebites and ethnobotany in the northwest región of Colombia. Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol. 2000, 73, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, J.C.; Martínez, D.; Quintana, J.C.; Jiménez, S.L.; Díaz, A.; Jiménez, I. Propagación in vitro de Renealmia alpinia (Rottb), planta con actividad antiofídica. Vitae 2008, 15, 61–69. [Google Scholar]
- Fernández, M.; Ortiz, W.; Pereáñez, J.; Martínez, D. Antiophidian properties evaluation of ethanol extract and fractions obtained from Renealmia alpinia (Rottb) Mass (Zingiberaceae) cultivated in vitro. Vitae 2010, 17, 75–82. [Google Scholar]
- Gómez-Betancur, I.; Benjumea, D.; Patiño, A.; Jiménez, N.; Osorio, E. Inhibition of the toxic effects of Bothrops asper venom by pinostrobin, a flavanone isolated from Renealmia alpinia (Rottb.) MAAS. J. Ethnopharmacol. 2014, 155, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Patiño, A.C.; Benjumea, D.M.; Pereañez, J.A. Inhibition of venom serine proteinase and metalloproteinase activities by Renealmia alpinia (Zingiberaceae) extracts: Comparison of wild and in vitro propagated plants. J. Ethnopharmacol. 2013, 149, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Loría, G.D.; Rucavado, A.; Kamiguti, A.S.; Theakston, R.D.G.; Fox, J.W.; Alape, A.; Gutiérrez, J.M. Characterization of “basparin A”, a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Arch. Biochem. Biophys. 2003, 418, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.; Rucavado, A.; Mora, N.; Gutiérrez, J.M. Purification and characterization of BaH4, a hemorrhagic metalloproteinase from the venom of the snake Bothrops asper. Toxicon 2000, 38, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.G.; Ownby, C.L. Systemic hemorrhage induced by proteinase H from Crotalus adamanteus (eastern diamondback rattlesnake) venom. Toxicon 1997, 35, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Theakston, R.D.G.; Desmond, H.; Hutton, R.A. Systemic haemorrhage in rats induced by a haemorrhagic fraction from Bothrops jararaca venom. Toxicon 1991, 29, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Núñez, J.; Moura da Silva, A.M.; Rucavado, A.; Theakston, R.D.G.; Gutiérrez, J.M. Pulmonary hemorrhage induced by jararhagin, a metalloproteinase from Bothrops jararaca venom. Toxicol. Appl. Pharmacol. 2003, 193, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Aragón, F.; Gubensek, F. Characterization of thrombin-like proteinase from Bothrops asper venom. In Toxins: Animal, Plant and Microbial; Rosenberg, P., Ed.; Pergamon Press: Oxford, UK, 1978; pp. 107–111. [Google Scholar]
- Fox, J.W.; Serrano, S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S. Platelets as targets of snake venom metalloproteinases. Toxicon 2005, 45, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Desmond, H.P.; Theakston, R.D.G.; Hay, C.R.M.; Zuzel, M. Ineffectiveness of the inhibition of the main haemorrhagic metalloproteinase from Bothrops jararaca venom by its only plasma inhibtor, a2-macroglobulin. Biochim. Biophys. Acta 1994, 1200, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Soto, M.; Escalante, T.; Loría, G.D.; Arni, R.; Gutiérrez, J.M. Thrombocytopenia and platelet hypoggregation induced by Bothrops asper snake venom: Toxins involved and their contribution to metalloproteinase-induced pulmonary hemorrhage. Thromb. Haemost. 2005, 94, 123–131. [Google Scholar] [PubMed]
- Lognay, G.; Marlier, M.; Severin, M.; Haugruge, E.; Gibon, V.; Trevejo, E. On the characterization of some terpenes from Renealmia alpinia Rottb. (Maas) Oleoresin. Flavour Frag. J. 1991, 6, 87–91. [Google Scholar] [CrossRef]
- Yang, S.W.; Zhou, B.N.; Malone, S.; Werkhoven, M.C.; van Troon, F.; Wisse, J.H.; Kingston, D.G. A new labdane diterpenoid from Renealmia alpinia collected in the Suriname rainforest. J. Nat. Prod. 1999, 62, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.N.; Baj, N.J.; Glass, T.E.; Malone, S.; Werkhoven, M.C.; van Troon, F.; David; Wisse, J.H.; Kingston, D.G. Bioactive labdane diterpenoids from Renealmia alpinia collected in the Suriname rainforest. J. Nat. Prod. 1997, 60, 1287–1293. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.O.; Fernandes, R.S.; Ticli, F.K.; Oliveira, C.Z.; Mazzi, M.V.; Franco, J.J.; Giuliatti, S.; Pereira, P.S.; Soares, A.M.; Sampaio, S.V. Triterpenoid saponins, new metalloprotease snake venom inhibitors isolated from Pentaclethra macroloba. Toxicon 2007, 50, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Pochet, L.; Frédérick, R.; Masereel, B. Coumarin and isocoumarin as serine protease inhibitors. Curr. Pharm. Des. 2004, 10, 3781–3796. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Tokiwa, T. Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells. Biol. Pharm. Bull. 2010, 33, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Patiño, A.C.; López, J.; Aristizábal, M.; Quintana, J.C.; Benjumea, D. Evaluation of the inhibitory effect of extracts from leaves of Renealmia alpinia Rottb. Maas (Zingiberaceae) on the venom of Bothrops asper (mapaná). Biomedica 2012, 32, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; pp. 141–173. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patiño, A.C.; Quintana, J.C.; Gutiérrez, J.M.; Rucavado, A.; Benjumea, D.M.; Pereañez, J.A. Extracts of Renealmia alpinia (Rottb.) MAAS Protect against Lethality and Systemic Hemorrhage Induced by Bothrops asper Venom: Insights from a Model with Extract Administration before Venom Injection. Toxins 2015, 7, 1532-1543. https://doi.org/10.3390/toxins7051532
Patiño AC, Quintana JC, Gutiérrez JM, Rucavado A, Benjumea DM, Pereañez JA. Extracts of Renealmia alpinia (Rottb.) MAAS Protect against Lethality and Systemic Hemorrhage Induced by Bothrops asper Venom: Insights from a Model with Extract Administration before Venom Injection. Toxins. 2015; 7(5):1532-1543. https://doi.org/10.3390/toxins7051532
Chicago/Turabian StylePatiño, Arley Camilo, Juan Carlos Quintana, José María Gutiérrez, Alexandra Rucavado, Dora María Benjumea, and Jaime Andrés Pereañez. 2015. "Extracts of Renealmia alpinia (Rottb.) MAAS Protect against Lethality and Systemic Hemorrhage Induced by Bothrops asper Venom: Insights from a Model with Extract Administration before Venom Injection" Toxins 7, no. 5: 1532-1543. https://doi.org/10.3390/toxins7051532






