Bioactive Components in Fish Venoms
Abstract
:1. Introduction

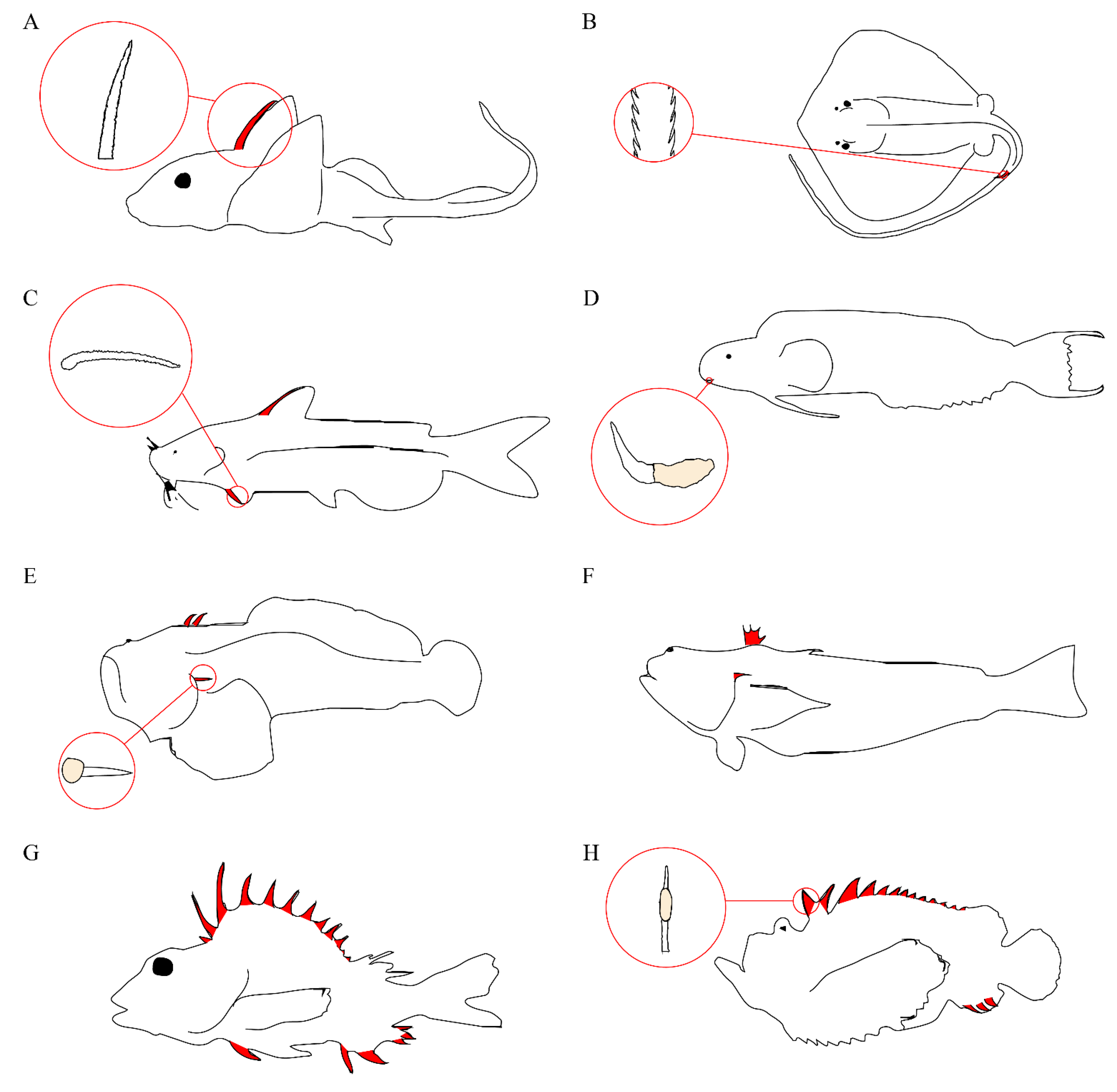
2. Venom Activities
2.1. Cardiovascular and Respiratory Systems
| Species | Pressure Effects | Model | Target | Source |
|---|---|---|---|---|
| Arius thallasinus | – | Rats, guinea pigs | Thulesius et al. [67] | |
| Trachinus draco | −/+/− | Rabbit | Evans [66] | |
| −/+/− | Cats | Evans [66] | ||
| Scatophagus argus | − | Cat, guinea pig | Histaminergic receptor | Muhuri et al. [69] |
| Pterois volitans | +/− | Aneasthetised rats | Muscarinic receptors | Church and Hodgson [70] |
| − (low dose), −/+ (high dose) | Anaesthetised rabbits | Saunders and Taylor [62] | ||
| Scorpaena guttata | Left ventricular end-diastolic pressure | Anaesthetized dog | Carlson et al. [63] | |
| + | Anaesthetized dog, pulmonary artery | Carlson et al. [63] | ||
| −/+ | Anaesthetized dog, systemic artery | Muscarinic receptors | Carlson et al. [63] | |
| Scorpaena plumieri | +(low dose), +/− (lethal dose) | Anaesthetised rats | Adrenoceptors | Gomes et al. [71] |
| Gymnapistes marmoratus | −/+ | Anaesthetised rat | Church and Hodgson [64] | |
| Synanceia horrida | + | Anaesthetised rabbits | α1, β2 adrenoceptors and leukotriene receptors | Hopkins et al. [72] |
| ++/+/− | Anaesthetised rats | α1, β2 adrenoceptors and leukotriene receptors | Hopkins et al. [72] | |
| − | Rabbits | Saunders et al. [73] |
| Species | Inotropic Response | Chronotropic Response | Model | Target | Source |
|---|---|---|---|---|---|
| Scatopagus argus | − | − | Isolated guinea pig heart | Muhuri et al. [69] | |
| + | + | Isolated toad heart | Muhuri et al. [69] | ||
| Pterois volitans | −/+ | Rat paced left atria | β1-adrenoceptors | Church and Hodgson [70] | |
| + | Rat spontaneously beating right atria | β1-adrenoceptors | Church and Hodgson [70] | ||
| − | − | Isolated clam and frog heart | Cohen and Olek [74] | ||
| Scorpaena guttata | −/+ | −/+ | Rat atria | Muscarinic receptors and β-adrenoceptors | Carlson et al. [65] |
| Gymnapistes marmoratus | −/+ | −/+ | Isolated rat atria | Muscarinic receptors and β-adrenoceptors | Church and Hodgson [64], Hopkins and Hodgson [68] |
| Scorpaena plumieri | +/− | Anaesthetised rats | Gomes et al. [71] | ||
| + | + | Isolated rat heart | Gomes et al. [71] | ||
| Synanceia horrida | −/+ | + (lyophilized venom), − (milked venom) | Isolated rat atria | M2 Muscarinic receptors and β1-adrenoceptors | Church and Hodgson [29] |
| Species | Effect | Model | Apparent Cause | Source |
|---|---|---|---|---|
| Tracinus draco | Cell Depolarization | Rat brain particles | Increase in TTP+ outflow | Chhatwal and Dreyer [75] |
| Scatophagus argus | Postsynaptic blockage of electrically induced twitch response | Isolated chick biventer cervices preparation | - | Muhuri et al. [69] |
| Relaxation response | Isolated rat duodenum preparation | - | Muhuri et al. [69] | |
| Contractile response | Isolated rat duodenum preparation, rat fundal strip, and rat uterus | - | Muhuri et al. [69] | |
| Gymnapistes marmoratus | Contraction response | Guinea pig isolated ileum and longitudinal smooth muscle preparations | Released endogenous acetylcholine and cyclooxygenase metabolites acting at muscarinic receptors | Hopkins et al. [76] |
| Contractile response | Chick biventer cervices muscle | Cell membrane pore formation | Church et al. [77] | |
| Pterois volitans | Irregular muscular fibrillation and muscular blockade | Isolated neuromuscular preparation | Release of endogenous acetylcholine from presynaptic nerve terminal | Cohen and Olek [74] |
| Contractile response | Chick biventer cervices muscle | Cell membrane pore formation | Church et al. [77] | |
| Synanceia horrida | Contractile response | Guinea pig isolated ileum | Released endogenous substance P acting at NK1 receptor | Hopkins et al. [72] |
| Reduced twitch height and increased basal tension | Chick biventer cervisis muscle preparation | Church and Hodgson [29] | ||
| Contractile response | Chick biventer cervices muscle | Cell membrane pore formation | Church et al. [77] | |
| Synanceia verrucosa | Cell Depolarization | Frog atrial heart muscle | Ca+ influx | Sauviat et al. [78] |
2.2. Neuromuscular System
2.3. Cytolytic Activity
| Species | Chicken | Sheep | Human | Rabbit | Rats | Mice | Cattle | Guinea Pig | Horse | Roach, Perch, Pigeon, Ox |
|---|---|---|---|---|---|---|---|---|---|---|
| Arius maculatus | + | + | + | |||||||
| Trachinus draco | X | + | + | + | + | + | + | + | + | + |
| Scatophagus argus | + | |||||||||
| Pterois antennata | X | X | + | X | X | X | ||||
| Pterois volitans | X | X | + | X | X | X | ||||
| Pterois lunulata | X | X | + | X | X | X | ||||
| Dendrochirus zebra | X | X | + | X | X | X | ||||
| Hypodytes rubripinnis | + | |||||||||
| Scorpaena guttata | + | |||||||||
| Notesthes robusta | + | |||||||||
| Inimicus japonicus | X | X | + | X | X | X | ||||
| Synanceia horrida | + | + | + | + | + | |||||
| Synanceia verrucosa | X | X | + | X | + | X |
2.4. Enzymatic Activity
| Species | Casein | Gelatin | Fibrinogen |
|---|---|---|---|
| Dasyatis guttata | + | + | + |
| Potamotrygon falkneri | + | + | + |
| Potamotrygon henlei | + | ||
| Potamotrygon scobina | + | ||
| Potamotrygon orbygnyi | + | ||
| Plesiotrygon iwamae | + | ||
| Arius thallasinus | + | ||
| Arius maculatus | + | + | |
| Thalassophryne nattereri | + | + | |
| Thalassophryne maculosa | + | ||
| Scatophagus argus | + | ||
| Scorpaena plumieri | + | + | |
| Pterois volitans | + | ||
| Notesthes robusta | + | ||
| Synanceia horrida | X | X |
2.5. Nociceptive, Edematic, and Necrotic Activities
2.6. Immune System Modulation
3. Venom Components
3.1. Proteinaceous Toxins
| Species | Toxin | MW | Source |
|---|---|---|---|
| Synanceia horrida (trachynis) | Trachynilysin (TLY) | 158 kDa (2 subunits) | Colasante et al. [128] |
| Stonustoxin (SNTX) | 148 kDa (2 subunits) | Poh et al. [31] | |
| Synanceia verrucosa | Verrucotoxin (VTX) | 322 kDa (4 subunits) | Garnier et al. [94] |
| Neoverrucotoxin (neoVTX) | 166 kDa (2 subunits) | Ueda et al. [129] | |
| Cardioleputin | 46 kDa | Abe et al. [130] | |
| Notesthes robusta | Nocitoxin | 169.8–174.5 kDa | Hahn and O’Connor [52] |
| Pterois volitans | * | 2 subunits, both ~75 kDa | Kiriake and Shiomi [131] |
| Pterois antennata | * | 2 subunits, both ~75 kDa | Kiriake and Shiomi [131] |
| Pterois lunulata | * | 160 kDa (2 subunits) | Kiriake et al. [87] |
| Inimicus japonicus | * | 160 kDa (2 subunits) | Kiriake et al. [87] |
| Hypodytes rubripinnis | * | 160 kDa (2 subunits) | Kiriake et al. [87] |
| Karatoxin | 110 kDa (2 subunits) | Nagasaka et al. [132] | |
| Sebastapistes stongia | * | N/A | Chuang and Shiao [133] |
| Scorpaenopsis oxycephala | * | N/A | Chuang and Shiao [133] |
| Sebasticus marmoratus | * | N/A | Chaung and Shiao [133] |
| Dendrochirus zebra | * | N/A | Chaung and Shiao [133] |
| Scorpaena plumieri | Sp-CTx | 121 kDa (2 subunits) | Andrich et al. [134] |
| Plumieribetin | 14 kDa | Evangelista et al. [135] | |
| SP-CL 1-5 | 16.8–17 kDa | Andrich et al. [136] | |
| Trachinus draco | Dracotoxin | 105 kDa | Chhatwal and Dreyer [75] |
| Trachinus vipera | Trachinine | 324 kDa (4 subunits) | Perriere et al. [137] |
| Scatophagus argus | SA-HT | 18 kDa | Karmakar et al. [138] |
| Thalassophryne maculosa | TmC4-47.2 | Unknown | Sosa-Rosales et al. [100] |
| Thalassophryne nattereri | Nattectin | 15 kDa | Lopes-Ferreira et al. [101] |
| Plotosus canius | Toxin-PC | 15 kDa | Auddy et al. [139] |
| Cathrops spixii | Wap65 | 54 kDa | Ramos et al. [58] |

3.2. Enzymes

3.3. Bioactive Peptides
3.4. Non-proteinaceous Components
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, G. Venoms to Drugs: Venom as a Source for the Development of Human Therapeutics; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; Diochot, S.; Corzo, G. Structure and pharmacology of spider venom neurotoxins. Biochimie 2000, 82, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. CMLS 2006, 63, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Church, J.E.; Hodgson, W.C. The pharmacological activity of fish venoms. Toxicon 2002, 40, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Borondo, J.; Sanz, P.; Nogue, S.; Poncela, J.; Garrido, P.; Valverde, J. Fatal weeverfish sting. Hum. Exp. Toxicol. 2001, 20, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.E.; Panos, T.C.; Kang, L.W.; Warner, A.M.; Colket, T.C., III. Studies on the mechanism of death from stingray venom a report of two fatal cases. Am. J. Med. Sci. 1958, 235, 566–584. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Gable, W.D. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon 1989, 27, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Hadok, J.C. Fatal envenomation by jellyfish causing Irukandji syndrome. Med. J. Aust. 2002, 177, 362–363. [Google Scholar] [PubMed]
- Rice, R.D.; Halstead, B.W. Report of fatal cone shell sting by Conus geographus Linnaeus. Toxicon 1968, 5, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.; Lopes-Ferreira, M. Clinical and experimental studies regarding poisoning caused by a fish Thalassophryne nattereri (niquim). An. Bras. Dermatol. 2000, 75, 435–443. [Google Scholar]
- Carrijo, L.C.; Andrich, F.; de Lima, M.E.; Cordeiro, M.N.; Richardson, M.; Figueiredo, S.G. Biological properties of the venom from the scorpionfish Scorpaena plumieri and purification of a gelatinolytic protease. Toxicon 2005, 45, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Casewell, N.R.; Ali, S.A.; Jackson, T.N.; Vetter, I.; Dobson, J.S.; Cutmore, S.C.; Nouwens, A.; Lavergne, V.; Fry, B.G. A ray of venom: Combined proteomic and transcriptomic investigation of fish venom composition using barb tissue from the blue-spotted stingray Neotrygon kuhlii. J. Proteomics 2014, 109, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, M.E.P.; Grund, L.Z.; Orii, N.M.; Saraiva, T.C.; de Magalhães Lopes, C.A.; Lima, C.; Lopes-Ferreira, M. Analysis of the inflammatory reaction induced by the catfish (Cathorops spixii) venoms. Toxicon 2007, 49, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Wheeler, W.C. Venom evolution widespread in fishes: A phylogenetic road map for the bioprospecting of piscine venoms. J. Hered. 2006, 97, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J. Diversity, phylogenetic distribution, and origins of venomous catfishes. BMC Evol. Biol. 2009, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.-H. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 2012, 40, W569–W572. [Google Scholar] [CrossRef] [PubMed]
- Halstead, B.W.; Chitwood, M.J.; Modglin, F.R. The anatomy of the venom apparatus of the zebrafish, Pterois volitans (Linnaeus). Anat. Rec. 1955, 122, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Halstead, B.W.; Chitwood, M.J.; Modglin, F.R. Stonefish stings, and the venom apparatus of Synanceja horrida (Linnaeus). Trans. Am. Microsc. Soc. 1956, 75, 381–397. [Google Scholar] [CrossRef]
- Halstead, B.W.; Chitwood, M.J.; Modglin, F.R. The venom apparatus of the California scorpionfish, Scorpaena guttata Girard. Trans. Am. Microsc. Soc. 1955, 74, 145–158. [Google Scholar] [CrossRef]
- Fishelson, L. Histology and ultrastructure of the recently found buccal toxic gland in the fish Meiacanthus nigrolineatus (Blenniidae). Copeia 1974, 2, 386–392. [Google Scholar] [CrossRef]
- Portillo Strempel, A.; Herrera Ceballos, E. Histology of the venom gland of Trachinus draco (Actinopterygii, Trachinidae). Acta Zool. 2014, 95, 125–132. [Google Scholar] [CrossRef]
- Endean, R. A study of distribution, habitat, behaviour, venom apparatus, and venom of the stone-fish. Mar. Freshw. Res. 1961, 12, 177–190. [Google Scholar] [CrossRef]
- Pedroso, C.M.; Jared, C.; Charvet-Almeida, P.; Almeida, M.P.; Neto, D.G.; Lira, M.S.; Haddad, V.; Barbaro, K.C.; Antoniazzi, M.M. Morphological characterization of the venom secretory epidermal cells in the stinger of marine and freshwater stingrays. Toxicon 2007, 50, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Halstead, B.W.; Bunker, N.C. The venom apparatus of the ratfish, Hydrolagus colliei. Copeia 1952, 1952, 128–138. [Google Scholar] [CrossRef]
- Collette, B.B. A review of the venomous toadfishes, subfamily Thalassophryninae. Copeia 1966, 4, 846–864. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Grund, L.Z.; Lima, C. Thalassophryne nattereri fish venom: From the envenoming to the understanding of the immune system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, J.; Hodgson, W. Dose-dependent cardiovascular and neuromuscular effects of stonefish (Synanceja trachynis) venom. Toxicon 2000, 38, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Cheung, P.; Leong, I.; Ruggieri, G.D. A non-proteinaceous toxin from the venomous spines of the lionfish Pterois volitans (Linnaeus). Toxicon 1985, 23, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.; Yuen, R.; Khoo, H.; Chung, M.; Gwee, M.; Gopalakrishnakone, P. Purification and partial characterization of stonustoxin (lethal factor) from Synanceja horrida venom. Comp. Biochem. Physiol. B: Comp. Biochem. 1991, 99, 793–798. [Google Scholar] [CrossRef]
- Smith, D. Order Saccopharyngiformes Cyematidae. In The Living Marine Resources of the Western Central Pacific: Batoid fishes, Chimaera and Bony Fishes Part 1 (Elopidae to Linophrynidae); FAO: Rome, Italy, 1999; Volume 3, p. 1693. [Google Scholar]
- Raju, S. 3 new species of genus Monognathus and Leptocephali of order Saccopharyngiformes. Fish. Bull. 1974, 72, 547–562. [Google Scholar]
- Smith, J. A case of poisoning by the stonefish, Synanceja verrucosa. Copeia 1951, 1951, 207–210. [Google Scholar] [CrossRef]
- Fitzgerald, G.J. Analysis of 24 cases of bullrout envenomation. Emerg. Med. 1993, 5, 199–200. [Google Scholar] [CrossRef]
- Kizer, K.W.; McKinney, H.E.; Auerbach, P.S. Scorpaenidae envenomation: A five-year poison center experience. JAMA 1985, 253, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Teoh, L.; Leo, S. Stonefish envenomations of the hand-a local marine hazard: A series of 8 cases and review of the literature. Ann. Acad. Med. Singap. 2004, 33, 515–520. [Google Scholar] [PubMed]
- Nistor, A.; Giè, O.; Biegger, P.; Fusetti, C.; Lucchina, S. Surgical vacuum-assisted closure for treatment of dramatic case of stonefish envenomation. Chin. J. Traumatol. 2010, 13, 250–252. [Google Scholar] [PubMed]
- Haddad, V., Jr.; Martins, I.A. Frequency and gravity of human envenomations caused by marine catfish (suborder siluroidei): A clinical and epidemiological study. Toxicon 2006, 47, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Wells, S. Lionfish envenomation of the hand. J. Hand Surg. 1993, 18, 523–525. [Google Scholar] [CrossRef]
- Auerbach, P.S.; McKinney, H.E.; Rees, R.S.; Heggers, J.P. Analysis of vesicle fluid following the sting of the lionfish Pterois volitans. Toxicon 1987, 25, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Núñez, J.; Rucavado, A.; Farsky, S.H.; Lomonte, B.; Angulo, Y.; Gutiérrez, J.M. Skeletal muscle necrosis and regeneration after injection of Thalassophryne nattereri (niquim) fish venom in mice. Int. J. Exp. Pathol. 2001, 82, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K. Venomous fish stings in tropical northern Australia. Am. J. Emerg. Med. 2001, 19, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Murphey, D.K.; Septimus, E.J.; Waagner, D.C. Catfish-related injury and infection: Report of two cases and review of the literature. Clin. Infect. Dis. 1992, 14, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Haddad, V.; Martins, I.A.; Makyama, H.M. Injuries caused by scorpionfishes (Scorpaena plumieri Bloch, 1789 and Scorpaena brasiliensis Cuvier, 1829) in the Southwestern Atlantic Ocean (Brazilian coast): Epidemiologic, clinic and therapeutic aspects of 23 stings in humans. Toxicon 2003, 42, 79–83. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.M.; Hahn, S.T. An epidemiological study of bullrout (Notesthes robusta) envenomation on the north coast of NSW. Aust. Emerg. Nurs. J. 2001, 4, 16–18. [Google Scholar] [CrossRef]
- Muirhead, D. Applying pain theory in fish spine envenomation. SPUMS 2002, 32, 150–153. [Google Scholar]
- Patkin, M.; Freeman, D. Bullrout stings. Med. J. Aust. 1969, 2, 14–16. [Google Scholar] [PubMed]
- Lopes-Ferreira, M.; Emim, J.A.D.S.; Oliveira, V.; Puzer, L.; Cezari, M.H.; Araújo, M.D.S.; Juliano, L.; Lapa, A.J.; Souccar, C.; Moura-da-Silva, A.M. Kininogenase activity of Thalassophryne nattereri fish venom. Biochem. Pharmacol. 2004, 68, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.L.; Menezes, T.N.; Carnielli, J.B.; Andrich, F.; Evangelista, K.S.; Chávez-Olórtegui, C.; Vassallo, D.V.; Figueiredo, S.G. Stonefish antivenom neutralises the inflammatory and cardiovascular effects induced by scorpionfish Scorpaena plumieri venom. Toxicon 2011, 57, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Hosaka, M.; Fujita, S.; Yamanaka, H.; Kikuchi, T. Venoms from six species of marine fish: Lethal and hemolytic activities and their neutralization by commercial stonefish antivenom. Mar. Biol. 1989, 103, 285–289. [Google Scholar] [CrossRef]
- Hahn, S.; O’connor, J. An investigation of the biological activity of bullrout (Notesthes robusta) venom. Toxicon 2000, 38, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Moura-da-Silva, A.; Mota, I.; Takehara, H. Neutralization of Thalassophryne nattereri (niquim) fish venom by an experimental antivenom. Toxicon 2000, 38, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Piran-Soares, A.A.; Souza, V.M.O.; Fonseca, L.A.; Lima, C.; Lopes-Ferreira, M. Neutralizing antibodies obtained in a persistent immune response are effective against deleterious effects induced by the Thalassophryne nattereri fish venom. Toxicon 2007, 49, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Halstead, B.W.; Courville, D.A. Poisonous and Venomous Marine Animals of the World: Vertebrates Continued; US Government Printing Office: Washington, DC, USA, 1970.
- Cameron, A.M.; Endean, R. Epidermal secretions and the evolution of venom glands in fishes. Toxicon 1973, 11, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Prithiviraj, N. Bioactive Properties of the Stone Fish Synanceia horrida Thomas 1984 Spine Venom. Int. J. Pharm. Biol. Sci. Arch. 2012, 3, 1217–1221. [Google Scholar]
- Ramos, A.D.; Conceição, K.; Silva, P.I., Jr.; Richardson, M.; Lima, C.; Lopes-Ferreira, M. Specialization of the sting venom and skin mucus of Cathorops spixii reveals functional diversification of the toxins. Toxicon 2012, 59, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Low, K.S.; Gwee, M.E.; Yuen, R.; Gopalakrishnakone, P.; Khoo, H. Stonustoxin: A highly potent endothelium-dependent vasorelaxant in the rat. Toxicon 1993, 31, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Skeie, E. Toxin of the Weeverfish (Trachinus draco). Acta Pharmacol. Toxicol. 1962, 19, 107–120. [Google Scholar] [CrossRef]
- Russell, F.E.; Emery, J.A. Venom of the weevers Trachinus draco and Trachinus vipera. Ann. N. Y. Acad. Sci. 1960, 90, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.R.; Taylor, P.B. Venom of the lionfish Pterois volitans. Am. J. Physiol. 1959, 197, 437–440. [Google Scholar] [PubMed]
- Carlson, R.; Schaeffer, R., Jr.; Whigham, H.; Weil, M.; Russell, F. Some pharmacological properties of the venom of the scorpionfish Scorpaena guttata—II. Toxicon 1973, 11, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Church, J.E.; Hodgson, W.C. Stonefish (Synanceia spp.) antivenom neutralises the in vitro and in vivo cardiovascular activity of soldierfish (Gymnapistes marmoratus) venom. Toxicon 2001, 39, 319–324. [Google Scholar]
- Carlson, R.W.; Schaeffer, R.C., Jr.; La Grange, R.G.; Roberts, C.M.; Russell, F.E. Some pharmacological properties of the venom of the scorpionfish Scorpaena guttata—I. Toxicon 1971, 9, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M. Observations on the poisoned spines of the weever fish (Trachinus draco). BMJ 1907, 1, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Thulesius, O.; Al-Hassan, J.M.; Criddle, R.S.; Thomson, M. Vascular responses elicited by venom of Arabian catfish (Arius thallasinus). Gen. Pharmacol.-Vasc. Syst 1983, 14, 129–132. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Hodgson, W.C. Cardiovascular studies on venom from the soldierfish (Gymnapistes marmoratus). Toxicon 1998, 36, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Muhuri, D.; Karmakar, S.; Dasgupta, S.; Nagchaudhuri, A.; Gomes, A. Pharmacological studies on the venomous spotted butterfish (Scatophagus argus Linn) sting extract on experimental animals. Indian J. Exp. Biol. 2004, 42, 461–467. [Google Scholar] [PubMed]
- Church, J.E.; Hodgson, W.C. Adrenergic and cholinergic activity contributes to the cardiovascular effects of lionfish (Pterois volitans) venom. Toxicon 2002, 40, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.L.; Andrich, F.; Mauad, H.; Sampaio, K.N.; de Lima, M.E.; Figueiredo, S.G.; Moysés, M.R. Cardiovascular effects of scorpionfish (Scorpaena plumieri) venom. Toxicon 2010, 55, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.J.; Hodgson, W.C.; Sutherland, S.K. Evidence for adrenergic and tachykinin activity in venom of the stonefish (Synanceja trachynis). Toxicon 1996, 34, 541–554. [Google Scholar] [CrossRef]
- Saunders, P.R.; Rothman, S.; Medrano, V.A.; Chin, H. Cardiovascular actions of venom of the stonefish Synanceja horrida. Am. J. Physiol. 1962, 203, 429–432. [Google Scholar] [PubMed]
- Cohen, A.S.; Olek, A.J. An extract of lionfish (Pterois volitans) spine tissue contains acetylcholine and a toxin that affects neuromuscular transmission. Toxicon 1989, 27, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Chhatwal, I.; Dreyer, F. Isolation and characterization of dracotoxin from the venom of the greater weever fish Trachinus draco. Toxicon 1992, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.J.; Hodgson, W.C.; Sutherland, S.K. An in vitro pharmacological examination of venom from the soldierfish Gymnapistes marmoratus. Toxicon 1997, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Church, J.E.; Moldrich, R.X.; Beart, P.M.; Hodgson, W.C. Modulation of intracellular Ca2+ levels by Scorpaenidae venoms. Toxicon 2003, 41, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Sauviat, M.-P.; Garnier, P.; Goudey-Perriere, F.; Perriere, C. Does crude venom of the stonefish (Synanceia verrucosa) activate β-adrenoceptors in the frog heart muscle? Toxicon 1995, 33, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Sivan, G.; Venketesvaran, K.; Radhakrishnan, C. Biological and biochemical properties of Scatophagus argus venom. Toxicon 2007, 50, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Lameiras, J.L.V.; Costa, O.T.F.D.; Moroni, F.T.; Araújo, J.D.R.; Caranhas, S.M.E.; Marques, C.M.A.; Dos-Santos, M.C.; Duncan, W.L.P. Systemic rhabdomyolysis induced by venom of freshwater stingrays Plesiotrygon iwamae and Potamotrygon motoro (Chondrichthyes–Potamotrygonidae) from the Amazon Basin. Toxicon 2014, 77, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Masson, A.; Ormonde do Carmo, P.; Carvalho, J. Rhabdomyolysis secondary to an accident with marine stingray (Dasyatis family). J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 344–348. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Thomson, M.; Ali, M.; Criddle, R.S. Toxic and pharmacologically active secretions from the Arabian Gulf catfish (Arius thalassinus, Ruppell). Toxin Rev. 1987, 6, 1–43. [Google Scholar] [CrossRef]
- Abirami, P.; Arumugam, M.; Giji, S.; Nagarajan, S. Bio-prospecting of catfish sting venom Arius maculatus available along South East coast of India. Int. J. Pharm. Pharm. Sci. 2014, 6, 110–115. [Google Scholar]
- Chhatwal, I.; Dreyer, F. Biological properties of a crude venom extract from the greater weever fish Trachinus draco. Toxicon 1992, 30, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.; Hon, W.; Lee, S.; Yuen, R. Effects of stonustoxin (lethal factor from Synanceja horrida venom) on platelet aggregation. Toxicon 1995, 33, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Duhig, J.; Jones, G. The venom apparatus of the stonefish (Synanceja horrida). Mem. Qld. Mus. 1928, 9, 136–150. [Google Scholar]
- Kiriake, A.; Suzuki, Y.; Nagashima, Y.; Shiomi, K. Proteinaceous toxins from three species of scorpaeniform fish (lionfish Pterois lunulata, devil stinger Inimicus japonicus and waspfish Hypodytes rubripinnis): Close similarity in properties and primary structures to stonefish toxins. Toxicon 2013, 70, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Grotendorst, G.R.; Hessinger, D.A. Purification and partial characterization of the phospholipase A 2 and co-lytic factor from sea anemone (Aiptasia pallida) nematocyst venom. Toxicon 1999, 37, 1779–1796. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Cruz, N.A.; Batista, C.V.; Possani, L.D. Phaiodactylipin, a glycosylated heterodimeric phospholipase A2 from the venom of the scorpion Anuroctonus phaiodactylus. Eur. J. Biochem. 2004, 271, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Smith, A.D. Haemolysis of intact human erythrocytes by purified cobra venom phospholipase A 2 in the presence of albumin and Ca 2+. BBA-Biomembr. 1974, 367, 271–281. [Google Scholar] [CrossRef]
- Ghafari, S.M.; Jamili, S.; Bagheri, K.P.; Ardakani, E.M.; Fatemi, M.R.; Shahbazzadeh, F.; Shahbazzadeh, D. The first report on some toxic effects of green scat, Scatophagus argus an Iranian Persian Gulf venomous fish. Toxicon 2013, 66, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Moura-da-Silva, A.M.; Piran-Soares, A.A.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.A.; Farsky, S.H. Hemostatic effects induced by Thalassophryne nattereri fish venom: A model of endothelium-mediated blood flow impairment. Toxicon 2002, 40, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.; Yuen, R.; Poh, C.; Tan, C. Biological activities of Synanceja horrida (stonefish) venom. Nat. Toxins 1992, 1, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.; Goudey-Perriere, F.; Breton, P.; Dewulf, C.; Petek, F.; Perriere, C. Enzymatic properties of the stonefish (Synanceia verrucosa Bloch and Schneider, 1801) venom and purification of a lethal, hypotensive and cytolytic factor. Toxicon 1995, 33, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, K.C.; Lira, M.S.; Malta, M.B.; Soares, S.L.; Garrone Neto, D.; Cardoso, J.L.; Santoro, M.L.; Haddad Junior, V. Comparative study on extracts from the tissue covering the stingers of freshwater (Potamotrygon falkneri) and marine (Dasyatis guttata) stingrays. Toxicon 2007, 50, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-dos-Santos, J.; Conceição, K.; Seibert, C.S.; Marques, E.E.; Silva, P.I., Jr.; Soares, A.B.; Lima, C.; Lopes-Ferreira, M. Studies on pharmacological properties of mucus and sting venom of Potamotrygon cf. henlei. Int. Immunopharmacol. 2011, 11, 1368–1377. [Google Scholar] [CrossRef]
- Antoniazzi, M.M.; Benvenuti, L.A.; Lira, M.S.; Jared, S.G.; Neto, D.G.; Jared, C.; Barbaro, K.C. Histopathological changes induced by extracts from the tissue covering the stingers of Potamotrygon falkneri freshwater stingrays. Toxicon 2011, 57, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, K.W.; Lima, C.; Piran-Soares, A.A.; Marques, E.E.; Hiruma-Lima, C.A.; Lopes-Ferreira, M. Biological and biochemical properties of the Brazilian Potamotrygon stingrays: Potamotrygon cf. scobina and Potamotrygon gr. orbignyi. Toxicon 2006, 47, 575–583. [Google Scholar]
- Lopes-Ferreira, M.; Barbaro, K.; Cardoso, D.; Moura-da-Silva, A.; Mota, I. Thalassophryne nattereri fish venom: Biological and biochemical characterization and serum neutralization of its toxic activities. Toxicon 1998, 36, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Rosales, J.I.; Piran-Soares, A.A.; Farsky, S.H.; Takehara, H.A.; Lima, C.; Lopes-Ferreira, M. Important biological activities induced by Thalassophryne maculosa fish venom. Toxicon 2005, 45, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Magalhaes, G.S.; Fernandez, J.H.; Junqueira-de-Azevedo, I.D.L.M.; le Ho, P.; Lima, C.; Valente, R.H.; Moura-da-Silva, A.M. Structural and biological characterization of Nattectin, a new C-type lectin from the venomous fish Thalassophryne nattereri. Biochimie 2011, 93, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Balasubashini, M.S.; Karthigayan, S.; Somasundaram, S.; Balasubramanian, T.; Viswanathan, P.; Menon, V.P. In vivo and in vitro characterization of the biochemical and pathological changes induced by lionfish (Pterios volitans) venom in mice. Toxicol. Mech. Method. 2006, 16, 525–531. [Google Scholar] [CrossRef]
- Cameron, A.M.; Surridge, J.; Stablum, W.; Lewis, R.J. A crinotoxin from the skin tubercle glands of a stonefish (Synanceia trachynis). Toxicon 1981, 19, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Sivan, G.; Venketasvaran, K.; Radhakrishnan, C. Characterization of biological activity of Scatophagus argus venom. Toxicon 2010, 56, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.J.; Hodgson, W.C. Enzyme and biochemical studies of stonefish (Synanceja trachynis) and soldierfish (Gymnapistes marmoratus) venoms. Toxicon 1998, 36, 791–793. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Tenório, H.; da Costa Marques, M.E.; Machado, S.S.; Pereira, H.J.V. Angiotensin processing activities in the venom of Talassophryne nattereri. Toxicon 2015, 98, 49–53. [Google Scholar]
- Austin, L.; Gillis, R.; Youatt, G. Stonefish venom: Some biochemical and chemical observations. Aust. J. Exp. Biol. Med. Sci. 1965, 43, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kiriake, A.; Madokoro, M.; Shiomi, K. Enzymatic properties and primary structures of hyaluronidases from two species of lionfish (Pterois antennata and Pterois volitans). Fish Physiol. Biochem. 2014, 40, 1043–1053. [Google Scholar] [PubMed]
- Magalhães, M.R. A hyaluronidase from Potamotrygon motoro (freshwater stingrays) venom: Isolation and characterization. Toxicon 2008, 51, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Ramos, A.D.; Martins, I.A.; Lima, C.; Conceição, K.; Haddad, V., Jr. Clinical manifestations and experimental studies on the spine extract of the toadfish Porichthys porosissimus. Toxicon 2014, 86, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sivan, G.; Radhakrishnan, C. Renal Lysosomal Functions on Exposure to Scatophagus argus Venom in Experimental Mice. Toxicol. Mech. Method. 2007, 17, 519–526. [Google Scholar] [CrossRef]
- Magalhaes, G.; Lopes-Ferreira, M.; Junqueira-de-Azevedo, I.; Spencer, P.; Araújo, M.; Portaro, F.; Ma, L.; Valente, R.; Juliano, L.; Fox, J. Natterins, a new class of proteins with kininogenase activity characterized from Thalassophryne nattereri fish venom. Biochimie 2005, 87, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Menezes, T.N.; Carnielli, J.B.; Gomes, H.L.; Pereira, F.E.; Lemos, E.M.; Bissoli, N.S.; Lopes-Ferreira, M.; Andrich, F.; Figueiredo, S.G. Local inflammatory response induced by scorpionfish Scorpaena plumieri venom in mice. Toxicon 2012, 60, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Santos, A.; Oliveira Souza, V.M.; Bruni, F.M.; Sosa-Rosales, J.I.; Lopes-Ferreira, M.; Lima, C. Delayed polymorphonuclear leukocyte infiltration is an important component of Thalassophryne maculosa venom pathogenesis. Toxicon 2008, 52, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Santos, A.; Saraiva, T.C.; Costa, E.P.; Santos, M.F.; Zorn, T.T.; Souza, V.M.O.; Lopes-Ferreira, M.; Lima, C. Delayed local inflammatory response induced by Thalassophryne nattereri venom is related to extracellular matrix degradation. Int. J. Exp. Pathol. 2009, 90, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Boletini-Santos, D.; Komegae, E.N.; Figueiredo, S.G.; Haddad, V., Jr.; Lopes-Ferreira, M.; Lima, C. Systemic response induced by Scorpaena plumieri fish venom initiates acute lung injury in mice. Toxicon 2008, 51, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Muhuri, D.; Dasgupta, S.; Gomes, A. Lethal, oedema, haemorrhagic activity of spotted butterfish (Scatophagus argus, Linn), sting extract and its neutralization by antiserum and pharmacological antagonists. Indian J. Exp. Biol. 2005, 43, 493. [Google Scholar] [PubMed]
- Lopes-Ferreira, M.; Gomes, E.M.; Bruni, F.M.; Ferreira, M.J.; Charvet, P.; Lima, C. First report of interruption of mast cell degranulation and endothelial cells activation by anti-inflammatory drugs controlling the acute response provoked by Pseudoplatystoma fasciatum fish venom. Toxicon 2014, 90, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kimura, L.F.; Prezotto-Neto, J.P.; Antoniazzi, M.M.; Jared, S.G.; Santoro, M.L.; Barbaro, K.C. Characterization of inflammatory response induced by Potamotrygon motoro stingray venom in mice. Exp. Biol. Med. 2014, 239, 601–609. [Google Scholar] [CrossRef]
- Lima, C.; Clissa, P.C.B.; Piran-Soares, A.A.; Tanjoni, I.; Moura-da-Silva, A.M.; Lopes-Ferreira, M. Characterisation of local inflammatory response induced by Thalassophryne nattereri fish venom in a mouse model of tissue injury. Toxicon 2003, 42, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, E.K.; Ferreira, M.J.; Grund, L.Z.; Coutinho, E.M.M.; Komegae, E.N.; Cassado, A.A.; Bortoluci, K.R.; Lopes-Ferreira, M.; Lima, C. Role of interplay between IL-4 and IFN-γ in the in regulating M1 macrophage polarization induced by Nattectin. Int. Immunopharmacol. 2012, 14, 513–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, T.C.; Grund, L.Z.; Komegae, E.N.; Ramos, A.D.; Conceicao, K.; Orii, N.M.; Lopes-Ferreira, M.; Lima, C. Nattectin a fish C-type lectin drives Th1 responses in vivo: Licenses macrophages to differentiate into cells exhibiting typical DC function. Int. Immunopharmacol. 2011, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Grund, L.Z.; Komegae, E.N.; Lopes-Ferreira, M.; Lima, C. IL-5 and IL-17A are critical for the chronic IgE response and differentiation of long-lived antibody-secreting cells in inflamed tissues. Cytokine 2012, 59, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. The hierarchical process of differentiation of long-lived antibody-secreting cells is dependent on integrated signals derived from antigen and IL-17A. PLoS One 2013, 8, e74566. [Google Scholar] [CrossRef] [PubMed]
- Grund, L.Z.; Souza, V.M.O.; Faquim-Mauro, E.L.; Lima, C.; Lopes-Ferreira, M. Experimental immunization with Thalassophryne nattereri fish venom: Striking IL-5 production and impaired of B220+ cells. Toxicon 2006, 48, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Komegae, E.N.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. The longevity of Th2 humoral response induced by proteases natterins requires the participation of long-lasting innate-like B cells and plasma cells in spleen. PLoS ONE 2013, 8, e67135. [Google Scholar] [CrossRef] [PubMed]
- Komegae, E.N.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. TLR2, TLR4 and the MyD88 signaling are crucial for the in vivo generation and the longevity of long-lived antibody-secreting cells. PLoS One 2013, 8, e71185. [Google Scholar] [CrossRef] [PubMed]
- Colasante, C.; Meunier, F.A.; Kreger, A.S.; Molgó, J. Selective depletion of clear synaptic vesicles and enhanced quantal transmitter release at frog motor nerve endings produced by trachynilysin, a protein toxin isolated from stonefish (Synanceia trachynis) venom. Eur. J. Neurosci. 1996, 8, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Suzuki, M.; Honma, T.; Nagai, H.; Nagashima, Y.; Shiomi, K. Purification, properties and cDNA cloning of neoverrucotoxin (neoVTX), a hemolytic lethal factor from the stonefish Synanceia verrucosa venom. BBA-Gen. Subj. 2006, 1760, 1713–1722. [Google Scholar] [CrossRef]
- Abe, T.; Sumatora, M.; Hashimoto, Y.; Yoshihara, J.; Shimamura, Y.; Fukami, J. Purification and properties of a cardioactive toxin, cardioleputin, from stonefish, Synanceja verrucosa. J. Venom. Anim. Toxins 1996, 2, 135–149. [Google Scholar] [CrossRef]
- Kiriake, A.; Shiomi, K. Some properties and cDNA cloning of proteinaceous toxins from two species of lionfish (Pterois antennata and Pterois volitans). Toxicon 2011, 58, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, K.; Nakagawa, H.; Satoh, F.; Hosotani, T.; Yokoigawa, K.; Sakai, H.; Sakuraba, H.; Ohshima, T.; Shinohara, M.; Ohura, K. A novel cytotoxic protein, Karatoxin, from the dorsal spines of the redfin velvetfish, Hypodytes rubripinnis. Toxin Rev. 2009, 28, 260–265. [Google Scholar] [CrossRef]
- Chuang, P.-S.; Shiao, J.-C. Toxin gene determination and evolution in scorpaenoid fish. Toxicon 2014, 88, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Andrich, F.; Carnielli, J.; Cassoli, J.; Lautner, R.; Santos, R.; Pimenta, A.; de Lima, M.; Figueiredo, S. A potent vasoactive cytolysin isolated from Scorpaena plumieri scorpionfish venom. Toxicon 2010, 56, 487–496. [Google Scholar] [CrossRef] [PubMed]
- De Santana Evangelista, K.; Andrich, F.; de Rezende, F.F.; Niland, S.; Cordeiro, M.N.; Horlacher, T.; Castelli, R.; Schmidt-Hederich, A.; Seeberger, P.H.; Sanchez, E.F. Plumieribetin, a fish lectin homologous to mannose-binding B-type lectins, inhibits the collagen-binding α1β1 integrin. J. Biol. Chem. 2009, 284, 34747–34759. [Google Scholar]
- Andrich, F.; Richardson, M.; Naumann, G.; Cordeiro, M.; Santos, A.; Santos, D.; Oliveira, J.; de Lima, M.; Figueiredo, S. Identification of C-type isolectins in the venom of the scorpionfish Scorpaena plumieri. Toxicon 2015, 95, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Perriere, C.; Goudey-Perriere, F.; Petek, F. Purification of a lethal fraction from the venom of the weever fish, Trachinus vipera CV. Toxicon 1988, 26, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Muhuri, D.; Dasgupta, S.; Nagchaudhuri, A.; Gomes, A. Isolation of a haemorrhagic protein toxin (SA-HT) from the Indian venomous butterfish (Scatophagus argus, Linn) sting extract. Indian J. Exp. Biol. 2004, 42, 452–460. [Google Scholar] [PubMed]
- Auddy, B.; Muhuri, D.C.; Alam, M.I.; Gomes, A. A lethal protein toxin (toxin-PC) from the Indian catfish (Plotosus canius, Hamilton) venom. Nat. Toxins 1995, 3, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.; Sauviat, M.-P.; Goudey-Perriere, F.; Perriere, C. Cardiotoxicity of verrucotoxin, a protein isolated from the venom of Synanceia verrucosa. Toxicon 1997, 35, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Wang, J.W.; Hao, L.Y.; Onoue, Y.; Kameyama, M. Verrucotoxin, a stonefish venom, modulates calcium channel activity in guinea-pig ventricular myocytes. Br. J. Pharmacol. 2007, 151, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.; Ducancel, F.; Ogawa, T.; Boulain, J.-C.; Goudey-Perrière, F.; Perrière, C.; Ménez, A. Complete amino-acid sequence of the β-subunit of VTX from venom of the stonefish (Synanceia verrucosa) as identified from cDNA cloning experiments. Biochim. Biophys. Acta 1997, 1337, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ghadessy, F.J.; Chen, D.; Kini, R.M.; Chung, M.C.; Jeyaseelan, K.; Khoo, H.E.; Yuen, R. Stonustoxin is a novel lethal factor from stonefish (Synanceja horrida) venom cDNA cloning and characterization. J. Biol. Chem. 1996, 271, 25575–25581. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Chen, D.; Yuen, R. Role of free thiol groups in the biological activities of stonustoxin, a lethal factor from stonefish (Synanceja horrida) venom. Toxicon 1998, 36, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kini, R.; Yuen, R.; Khoo, H. Haemolytic activity of stonustoxin from stonefish (Synanceja horrida) venom: Pore formation and the role of cationic amino acid residues. Biochem. J. 1997, 325, 685–691. [Google Scholar] [PubMed]
- Khoo, H.E.; Chen, D.; Yuen, R. The role of cationic amino acid residues in the lethal activity of stonustoxin from stonefish (Synanceja horrida) venom. IUBMB Life 1998, 44, 643–646. [Google Scholar] [CrossRef]
- Yuen, R.; Cai, B.; Khoo, H. Production and characterization of monoclonal antibodies against stonustoxin from Synanceja horrida. Toxicon 1995, 33, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.; Khoo, H.; Moore, P.; Bhatia, M.; Lu, J.; Moochhala, S. Synergism between hydrogen sulfide (H2S) and nitric oxide (NO) in vasorelaxation induced by stonustoxin (SNTX), a lethal and hypotensive protein factor isolated from stonefish Synanceja horrida venom. Life Sci. 2007, 80, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Sauviat, M.-P.; Meunier, F.A.; Kreger, A.; Molgó, J. Effects of trachynilysin, a protein isolated from stonefish (Synanceia trachynis) venom, on frog atrial heart muscle. Toxicon 2000, 38, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Meunier, F.A.; Mattei, C.; Chameau, P.; Lawrence, G.; Colasante, C.; Kreger, A.S.; Dolly, J.O.; Molgó, J. Trachynilysin mediates SNARE-dependent release of catecholamines from chromaffin cells via external and stored Ca2+. J. Cell Sci. 2000, 113, 1119–1125. [Google Scholar] [PubMed]
- Ouanounou, G.; Malo, M.; Stinnakre, J.; Kreger, A.S.; Molgó, J. Trachynilysin, a neurosecretory protein isolated from stonefish (Synanceia trachynis) venom, forms nonselective pores in the membrane of NG108–15 cells. J. Biol. Chem. 2002, 277, 39119–39127. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.L.; Andrich, F.; Fortes-Dias, C.L.; Perales, J.; Teixeira-Ferreira, A.; Vassallo, D.V.; Cruz, J.S.; Figueiredo, S.G. Molecular and biochemical characterization of a cytolysin from the Scorpaena plumieri (scorpionfish) venom: Evidence of pore formation on erythrocyte cell membrane. Toxicon 2013, 74, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Nagasaka, K.; Nakagawa, H.; Edo, K.; Sakai, H.; Kato, K.; Iwaki, F.; Ohura, K.; Sakuraba, H. A novel chemoattractant lectin, karatoxin, from the dorsal spines of the small scorpionfish Hypodytes rubripinnis. J. Pharmacol. Sci. 2010, 113, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Komegae, E.N.; Ramos, A.D.; Oliveira, A.K.; de Toledo Serrano, S.M.; Lopes-Ferreira, M.; Lima, C. Insights into the local pathogenesis induced by fish toxins: Role of natterins and nattectin in the disruption of cell–cell and cell–extracellular matrix interactions and modulation of cell migration. Toxicon 2011, 58, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, G.; Junqueira-de-Azevedo, I.; Lopes-Ferreira, M.; Lorenzini, D.; Ho, P.; Moura-da-Silva, A. Transcriptome analysis of expressed sequence tags from the venom glands of the fish Thalassophryne nattereri. Biochimie 2006, 88, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Lima, C.; Lopes-Ferreira, M. Anti-inflammatory effect of Natterins, the major toxins from the Thalassophryne nattereri fish venom is dependent on TLR4/MyD88/PI3K signaling pathway. Toxicon 2014, 87, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.; Yuen, R.; Chung, M.; Khoo, H. Purification and partial characterization of hyaluronidase from stonefish (Synanceja horrida) venom. Comp. Biochem. Physiol. B: Comp. Biochem. 1992, 101, 159–163. [Google Scholar] [CrossRef]
- Ng, H.C.; Ranganathan, S.; Chua, K.L.; Khoo, H.E. Cloning and molecular characterization of the first aquatic hyaluronidase, SFHYA1, from the venom of stonefish (Synanceja horrida). Gene 2005, 346, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Yamada, S.; Sugiura, M.; Takeda, K.; Yuen, R.; Khoo, H.; Poh, C. Identification of the reaction products of the purified hyaluronidase from stonefish (Synanceja horrida) venom. Biochem. J. 1992, 283, 99–104. [Google Scholar] [PubMed]
- Madokoro, M.; Ueda, A.; Kiriake, A.; Shiomi, K. Properties and cDNA cloning of a hyaluronidase from the stonefish Synanceia verrucosa venom. Toxicon 2011, 58, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Conceição, K.; Konno, K.; Melo, R.L.; Marques, E.E.; Hiruma-Lima, C.A.; Lima, C.; Richardson, M.; Pimenta, D.C.; Lopes-Ferreira, M. Orpotrin: A novel vasoconstrictor peptide from the venom of the Brazilian Stingray Potamotrygon gr. orbignyi. Peptides 2006, 27, 3039–3046. [Google Scholar] [CrossRef]
- Conceição, K.; Santos, J.M.; Bruni, F.M.; Klitzke, C.F.; Marques, E.E.; Borges, M.H.; Melo, R.L.; Fernandez, J.H.; Lopes-Ferreira, M. Characterization of a new bioactive peptide from Potamotrygon gr. orbignyi freshwater stingray venom. Peptides 2009, 30, 2191–2199. [Google Scholar]
- Sri Balasubashini, M.; Karthigayan, S.; Somasundaram, S.; Balasubramanian, T.; Viswanathan, V.; Raveendran, P.; Menon, V.P.. Fish venom (Pterios volitans) peptide reduces tumor burden and ameliorates oxidative stress in Ehrlich’s ascites carcinoma xenografted mice. Bioorg. Med. Chem. Lett. 2006, 16, 6219–6225. [Google Scholar]
- Carlisle, D. On the venom of the lesser weeverfish, Trachinus vipera. J. Mar. Biol. Assoc. U.K. 1962, 42, 155–162. [Google Scholar] [CrossRef]
- Garnier, P.; Grosclaude, J.-M.; Goudey-Perrière, F.; Gervat, V.; Gayral, P.; Jacquot, C.; Perrière, C. Presence of norepinephrine and other biogenic amines in stonefish venom. J. Chromotogr. B: Biomed. 1996, 685, 364–369. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Hodgson, W.C.; Sutherland, S.K. Pharmacological studies of stonefish (Synanceja trachynis) venom. Toxicon 1994, 32, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Davis, J.L.; Rash, L.D.; Anangi, R.; Mobli, M.; Alewood, P.F.; Lewis, R.J.; King, G.F. Venomics: A new paradigm for natural products-based drug discovery. Amino Acids 2011, 40, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Jin, A.-H.; Kaas, Q.; Jones, A.; Alewood, P.F.; Lewis, R.J. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol. Cell. Proteomics 2013, 12, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteomics 2014, 106, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.S.; Undheim, E.A.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider venomics: Implications for drug discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: Biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteomics 2014, 105, 323–339. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegman, R.; Alewood, P. Bioactive Components in Fish Venoms. Toxins 2015, 7, 1497-1531. https://doi.org/10.3390/toxins7051497
Ziegman R, Alewood P. Bioactive Components in Fish Venoms. Toxins. 2015; 7(5):1497-1531. https://doi.org/10.3390/toxins7051497
Chicago/Turabian StyleZiegman, Rebekah, and Paul Alewood. 2015. "Bioactive Components in Fish Venoms" Toxins 7, no. 5: 1497-1531. https://doi.org/10.3390/toxins7051497





