MC-LR Exposure Leads to Subfertility of Female Mice and Induces Oxidative Stress in Granulosa Cells
Abstract
:1. Introduction
2. Results
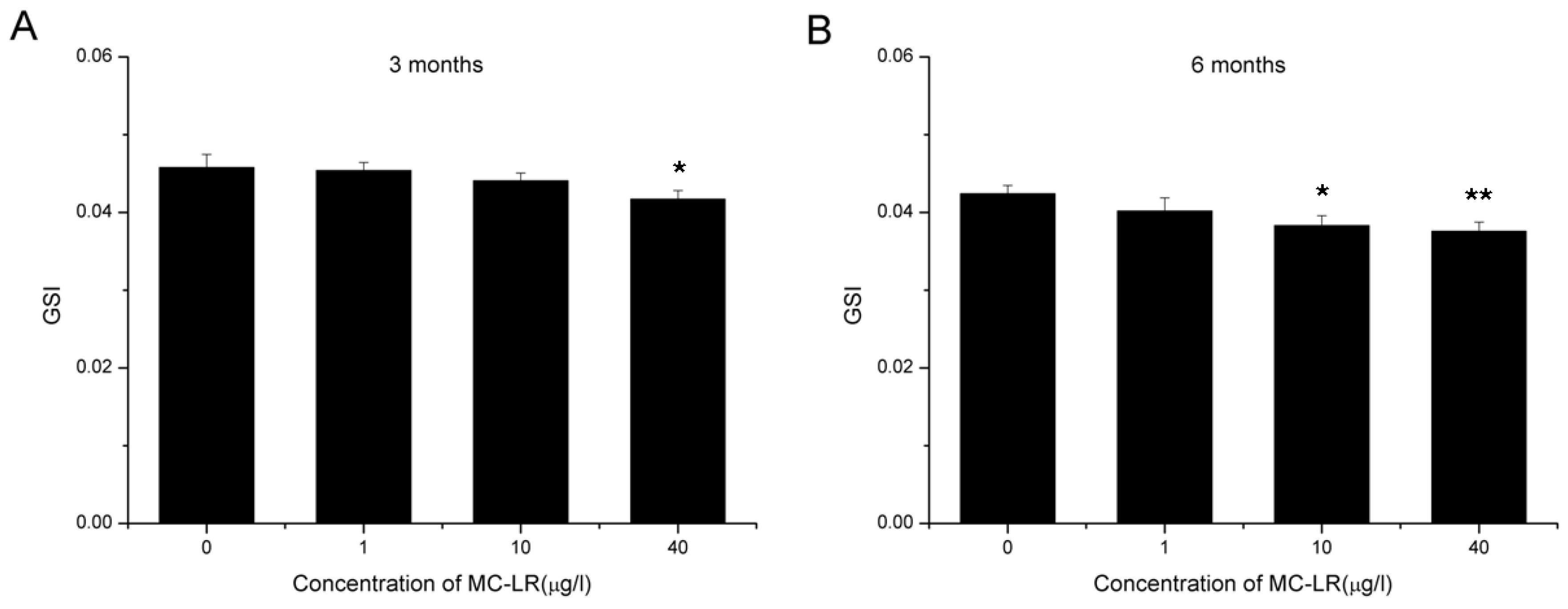
2.1. Reduction in GSI
2.2. Accelerated Follicle Atresia and Loss of Developing Follicles

2.3. Decline of Serum Estradiol and Ascending of Serum Progesterone

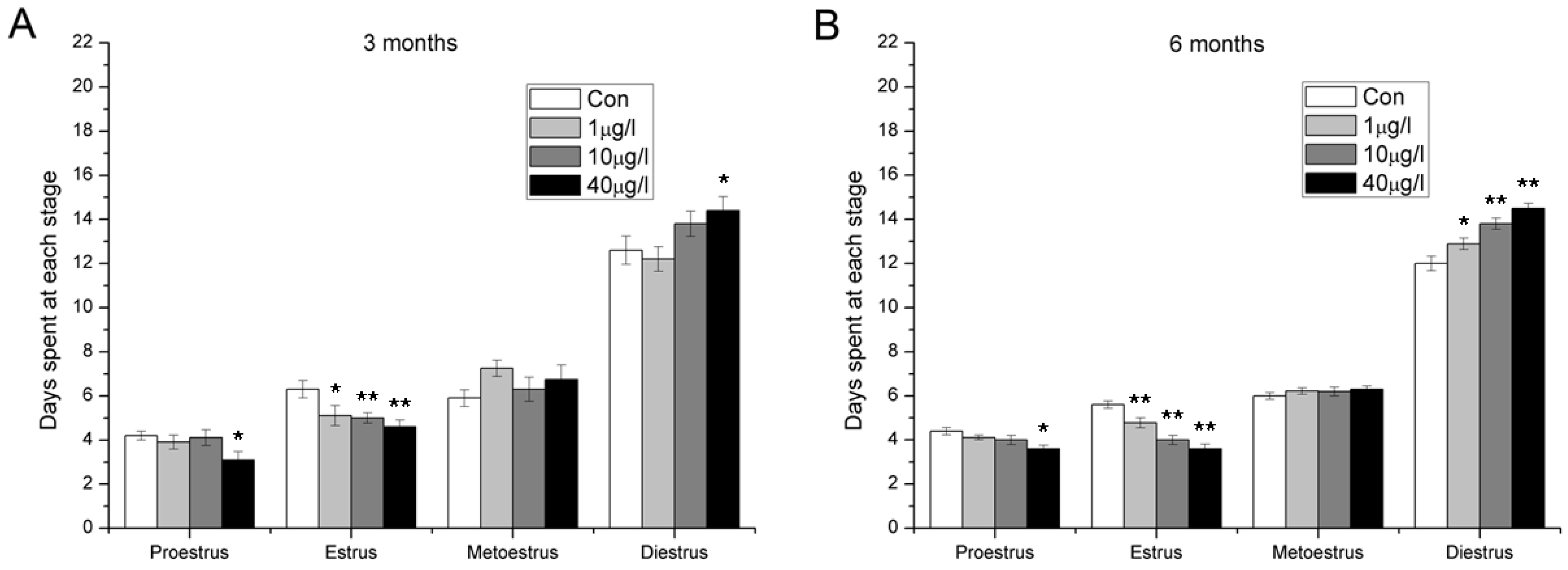
2.4. Disorder of Estrous Cycle
2.5. Subfertility Induced by MC-LR
| MC-LR (μg/L) | N | Litter | Living Pups | Dead Pups | Stillbirth Rate | Living Pups/Litter |
|---|---|---|---|---|---|---|
| 0 | 10 | 40 | 261 | 4 | 1.32 ± 0.62 | 6.53 ± 0.40 |
| 1 | 10 | 40 | 220 | 6 | 2.19 ± 0.95 | 5.50 ± 0.40 |
| 10 | 10 | 38 | 228 | 15 | 5.71 ± 1.75 * | 6.00 ± 0.48 |
| 40 | 10 | 34 | 171 | 15 | 8.63 ± 2.12 ** | 5.03 ± 0.64 * |
| MC-LR (μg/L) | N | Litter | Living Pups | Dead Pups | Stillbirth Rate | Living Pups/Litter |
|---|---|---|---|---|---|---|
| 0 | 10 | 36 | 168 | 4 | 1.75 ± 1.09 | 4.67 ± 0.51 |
| 1 | 10 | 28 | 117 | 8 | 4.59 ± 2.05 | 4.18 ± 0.54 |
| 10 | 10 | 34 | 112 | 14 | 10.20 ± 3.64 * | 3.29 ± 0.49 * |
| 40 | 10 | 32 | 106 | 15 | 9.18 ± 2.36 * | 3.31 ± 0.38 * |
2.6. MC-LR Affects Cell Viability of mGCs

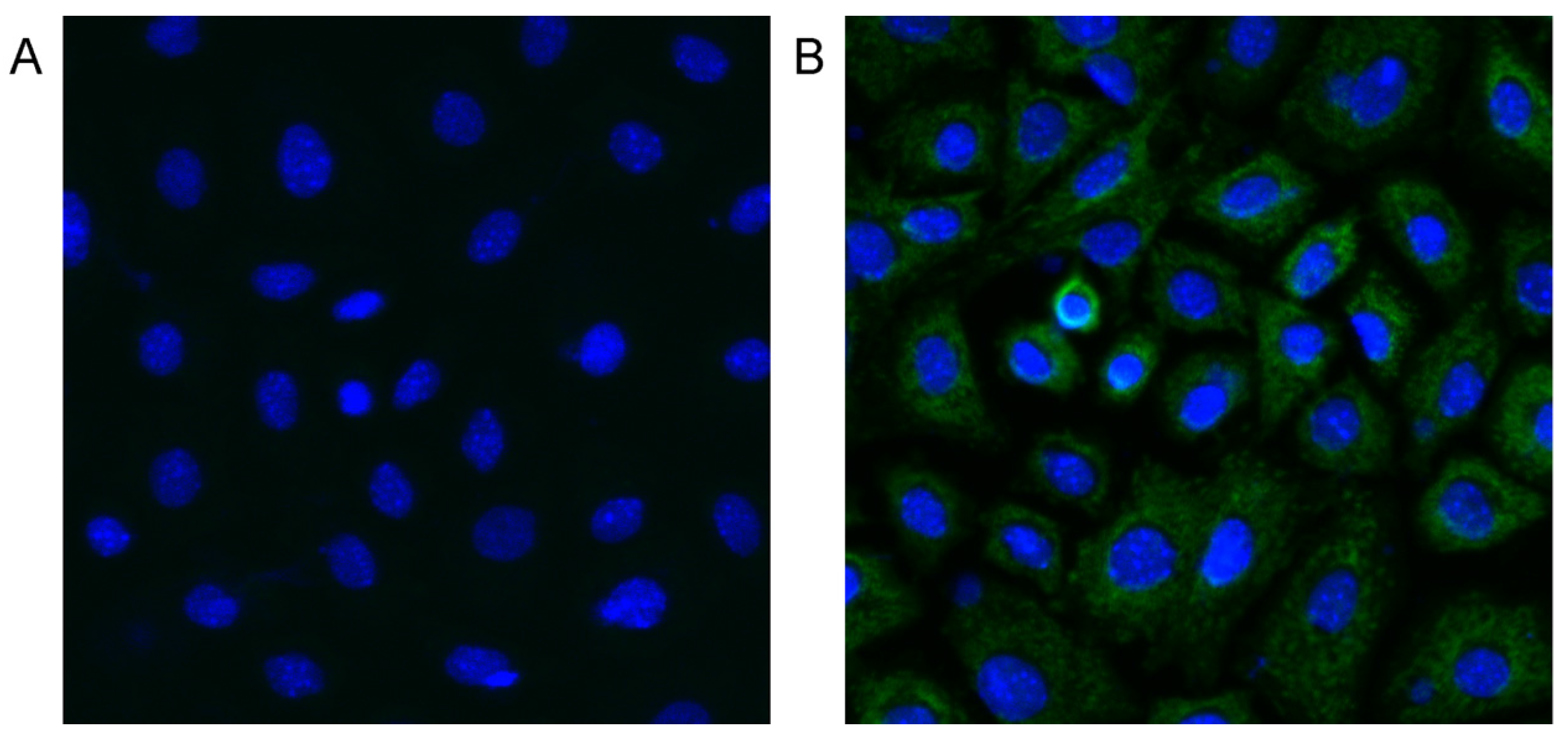
2.7. MC-LR can Enter mGCs
2.8. Increased Lipid Peroxidation and Inhibition of Antioxidant Enzymes Activity

3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Animals and Treatment
4.3. GSI Calculation
4.4. Histological Evaluation of Follicles
4.5. Estrous Cycle Monitoring
4.6. Serum Hormone Assay
4.7. Assessment of Fertility
4.8. Mouse Primary Granulosa Cell Collection and Culture
4.9. Cell Viability Assay
4.10. Immunofluorescence Detection
4.11. Determination of Lipid Peroxidation and Antioxidant Enzymes
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Dervaux, J.; Mejean, A.; Brunet, P. Irreversible collective migration of cyanobacteria in eutrophic conditions. PLoS ONE 2015, 10, e0120906. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Korak, J.A.; Trenholm, R.A.; Rosario-Ortiz, F.L. Effect of oxidant exposure on the release of intracellular microcystin, mib, and geosmin from three cyanobacteria species. Water Res. 2014, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Feurstein, D.; Stemmer, K.; Kleinteich, J.; Speicher, T.; Dietrich, D.R. Microcystin congener- and concentration-dependent induction of murine neuron apoptosis and neurite degeneration. Toxicol. Sci. 2011, 124, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Vesterkvist, P.S.; Misiorek, J.O.; Spoof, L.E.; Toivola, D.M.; Meriluoto, J.A. Comparative cellular toxicity of hydrophilic and hydrophobic microcystins on caco-2 cells. Toxins (Basel) 2012, 4, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.C.; Haney, J.F.; Cottingham, K.L. First report of microcystin-LR in the cyanobacterium Gloeotrichia echinulata. Environ. Toxicol. 2007, 22, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the midwestern united states. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Haddix, P.L.; Hughley, C.J.; Lechevallier, M.W. Occurrence of microcystins in 33 us water supplies. J. Am. Water Works Assoc. 2007, 99, 118–125. [Google Scholar]
- Izaguirre, G.; Jungblut, A.D.; Neilan, B.A. Benthic cyanobacteria (oscillatoriaceae) that produce microcystin-LR, isolated from four reservoirs in southern California. Water Res. 2007, 41, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Akcaalan, R.; Young, F.M.; Metcalf, J.S.; Morrison, L.F.; Albay, M.; Codd, G.A. Microcystin analysis in single filaments of Planktothrix spp. In laboratory cultures and environmental blooms. Water Res. 2006, 40, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Pitois, S.; Jackson, M.H.; Wood, B.J.B. Problems associated with the presence of cyanobacteria in recreational and drinking waters. Int. J. Environ. Health Res. 2000, 10, 203–218. [Google Scholar] [CrossRef]
- Van Apeldoorn, M.E.; van Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.; Vilarino, N.; Louzao, M.C.; Rodriguez, L.P.; Alfonso, A.; Campbell, K.; Elliott, C.T.; Taylor, P.; Ramos, V.; Vasconcelos, V.; et al. Multi-detection method for five common microalgal toxins based on the use of microspheres coupled to a flow-cytometry system. Anal. Chim. Acta 2014, 850, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Svirèev, Z.; Simeunoviè, J.; Subakov-Simiè, G.; Krstiè, S.; Vidoviè, M. Freshwater cyanobacterial blooms and cyanotoxin production in serbia in the past 25 years. Geogr. Pannonica 2007, 11, 32–28. [Google Scholar]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cyanobacterial toxins: Microcystin-LR. In Guidelines for Drinking-Water Quality, 2nd ed.; Addendum to Volume 2, Health Criteria and Other Supporting Information; WHO: Geneva, Swizerland, 1998; pp. 95–110. [Google Scholar]
- Blaha, L.; Marsalek, B. Contamination of drinking water in the czech republic by microcystins. Arch. Hydrobiol. 2003, 158, 421–429. [Google Scholar] [CrossRef]
- Gurbuz, F.; Metcalf, J.S.; Karahan, A.G.; Codd, G.A. Analysis of dissolved microcystins in surface water samples from kovada lake, Turkey. Sci. Total Environ. 2009, 407, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in salto grande dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y. The role of PP2A-associated proteins and signal pathways in microcystin-LR toxicity. Toxicol. Lett. 2015, 236. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The fate of microcystins in the environment and challenges for monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, S.J.; Zhou, F.; Wen, S.Y.; Chen, F.; Han, X.D. Reproductive toxicity on female mice induced by microcystin-LR. Environ. Toxicol. Pharmacol. 2014, 37. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. TGF-β superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Orisaka, M.; Wang, H.; Orisaka, S.; Thompson, W.; Zhu, C.; Kotsuji, F.; Tsang, B.K. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: The delicate balance between life and death. Front. Biosci. 2007, 12, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Prizant, H.; Light, A.; Biswas, A.; Hayes, E.; Lee, H.J.; Barad, D.; Gleicher, N.; Hammes, S.R. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Hanoch, T.; Rosenberg, R.; Dantes, A.; Merz, W.E.; Strauss, J.F., 3rd; Amsterdam, A. The erk signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2001, 276, 13957–13964. [Google Scholar] [PubMed]
- Tajima, K.; Dantes, A.; Yao, Z.; Sorokina, K.; Kotsuji, F.; Seger, R.; Amsterdam, A. Down-regulation of steroidogenic response to gonadotropins in human and rat preovulatory granulosa cells involves mitogen-activated protein kinase activation and modulation of DAX-1 and steroidogenic factor-1. J. Clin. Endocrinol. Metab. 2003, 88, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Furukawa, T.; Ikeda, R.; Takumi, S.; Nong, Q.; Aoyama, K.; Akiyama, S.; Keppler, D.; Takeuchi, T. Involvement of mitogen-activated protein kinase signaling pathways in microcystin-LR-induced apoptosis after its selective uptake mediated by OATP1B1 and OATP1B3. Toxicol. Sci. 2007, 97, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, D.; Tozer, A.J.; Docherty, S.M.; Iles, R.K. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod. Biol. Endocrinol. 2010, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.F. Sexual behavior in continuously cycling rats. Behaviour 1972, 41, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, K.; Lima, S.Q. Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr. Biol. 2015, 25, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, C.H. Timing of sexual behavior in the female rat. Endocrinology 1970, 86, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Daniel, W.S. Cyanobacteria toxins and the current state of knowledge on water treatment options: A review. J. Environ. Eng. Sci. 2004, 3, 155–185. [Google Scholar]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Steiner, K.; Zimmermann, L.; Hagenbuch, B.; Dietrich, D. Zebrafish OATP-mediated transport of microcystin congeners. Arch. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Pichardo, S.; Jos, A.; Camean, A.M. Oxidative stress induced by microcystin-LR on PLHC-1 fish cell line. Toxicol. In Vitro 2009, 23, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Pichardo, S.; Jos, A.; Prieto, A.I.; Sevilla, E.; Frias, J.E.; Camean, A.M. Differential oxidative stress responses to pure microcystin-LR and microcystin-containing and non-containing cyanobacterial crude extracts on Caco-2 cells. Toxicon 2010, 55, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ros in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Roegner, A.F.; Brena, B.; Gonzalez-Sapienza, G.; Puschner, B. Microcystins in potable surface waters: Toxic effects and removal strategies. J. Appl. Toxicol. 2014, 34, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Britt, K.L.; Wreford, N.G.; Ebling, F.J.; Kerr, J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kipp, J.L.; Kilen, S.M.; Woodruff, T.K.; Mayo, K.E. Activin regulates estrogen receptor gene expression in the mouse ovary. J. Biol. Chem. 2007, 282, 36755–36765. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Yuan, M.; Song, Y.; Sun, F.; Han, X. MC-LR Exposure Leads to Subfertility of Female Mice and Induces Oxidative Stress in Granulosa Cells. Toxins 2015, 7, 5212-5223. https://doi.org/10.3390/toxins7124872
Wu J, Yuan M, Song Y, Sun F, Han X. MC-LR Exposure Leads to Subfertility of Female Mice and Induces Oxidative Stress in Granulosa Cells. Toxins. 2015; 7(12):5212-5223. https://doi.org/10.3390/toxins7124872
Chicago/Turabian StyleWu, Jiang, Mingming Yuan, Yuefeng Song, Feng Sun, and Xiaodong Han. 2015. "MC-LR Exposure Leads to Subfertility of Female Mice and Induces Oxidative Stress in Granulosa Cells" Toxins 7, no. 12: 5212-5223. https://doi.org/10.3390/toxins7124872





