Oxidized Lipids and Lysophosphatidylcholine Induce the Chemotaxis, Up-Regulate the Expression of CCR9 and CXCR4 and Abrogate the Release of IL-6 in Human Monocytes
Abstract
:1. Introduction
2. Results
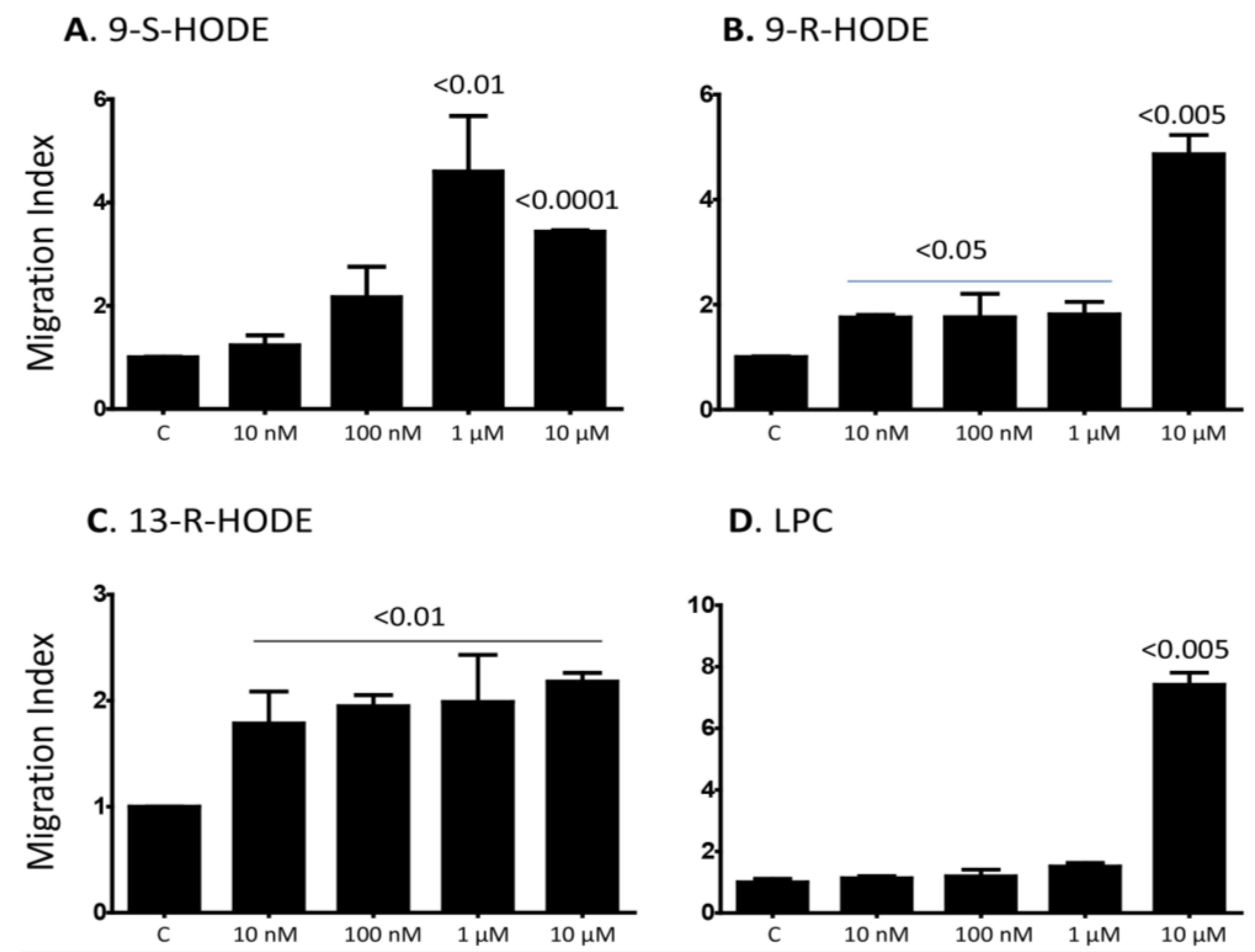
2.1. Several Isoforms of HODEs and LPC Induce Chemotaxis of Primary Human Monocytes
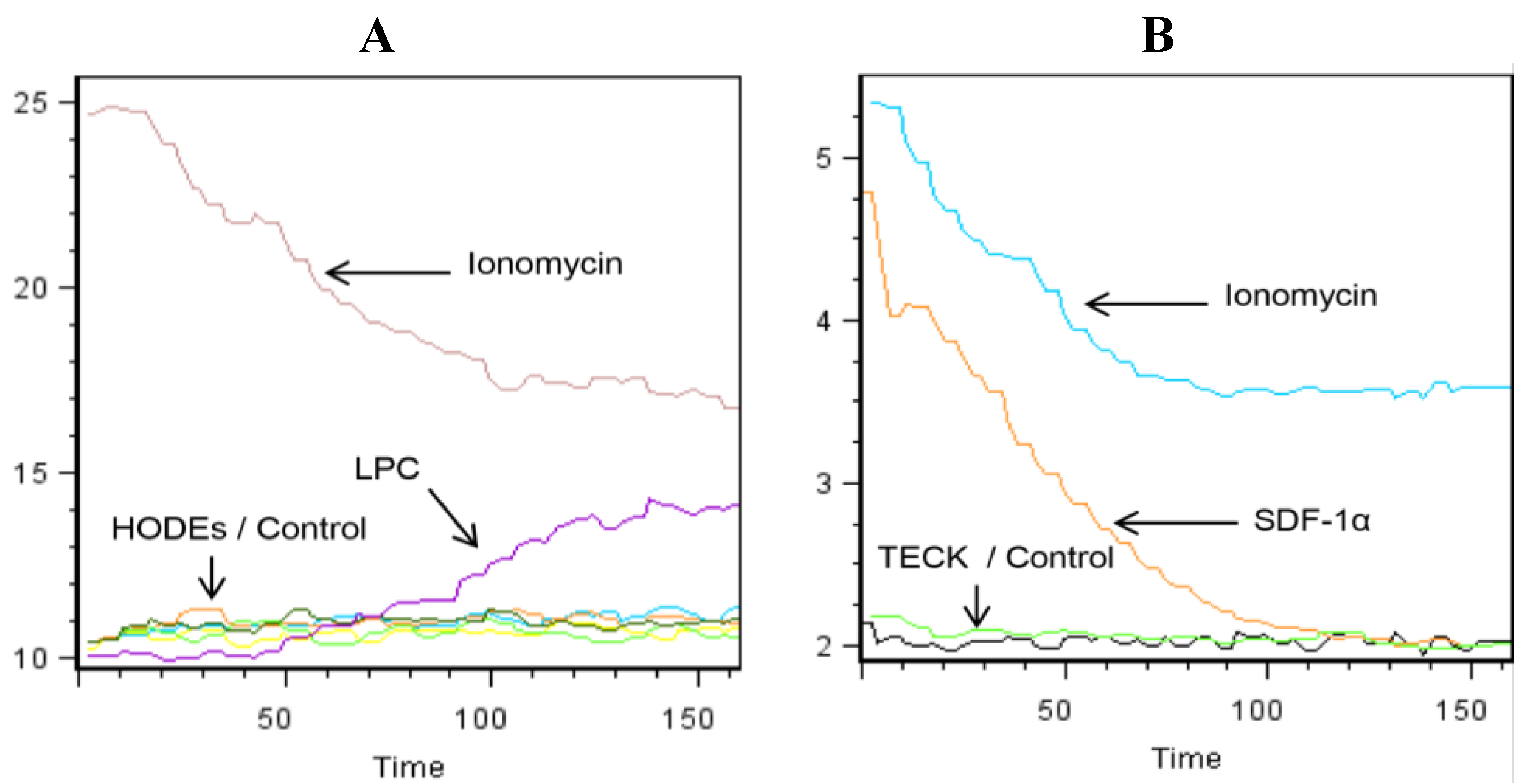
2.2. LPC Induces the Mobilization of Intracellular Calcium in Primary Human Monocytes
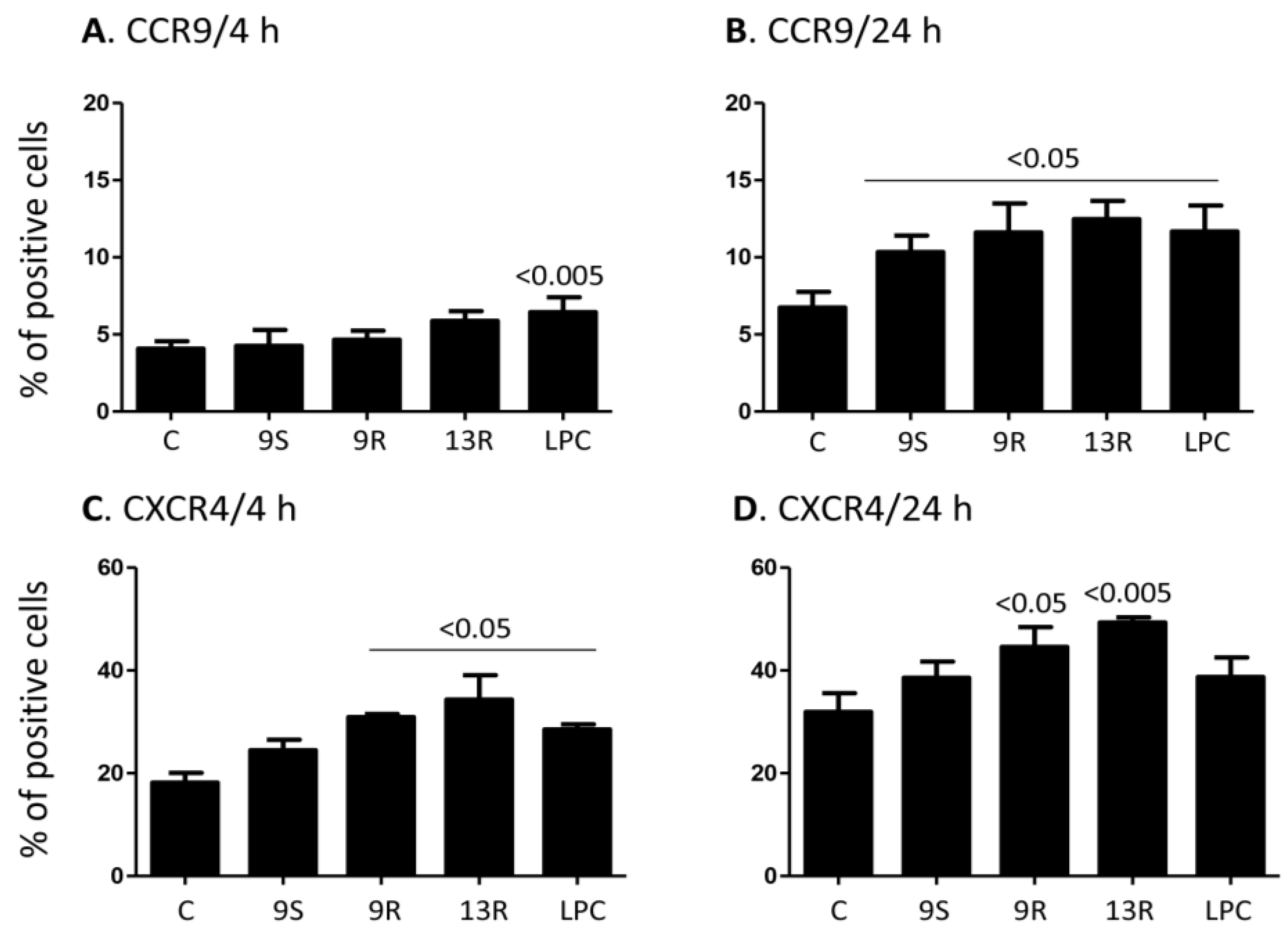
2.3. Oxidized Lipids and LPC Increase the Expression of CCR9 and CXCR4 on the Surface of Monocytes
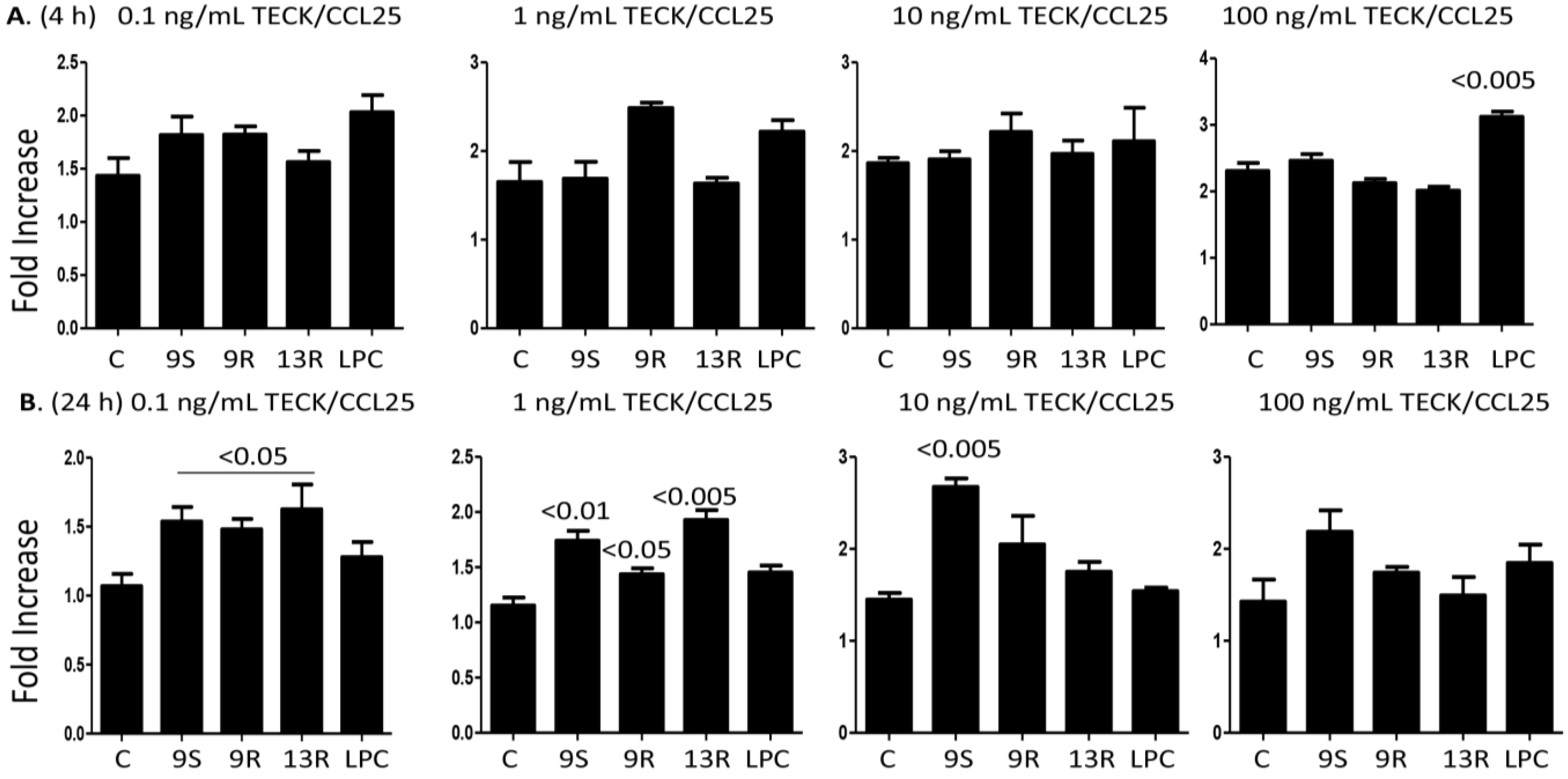
2.4. Oxidized Lipids and LPC Augment Monocyte Chemotaxis towards TECK/CCL25
2.5. Oxidized Lipids and LPC Induce Increased Chemotaxis towards SDF-1α/CXCL12
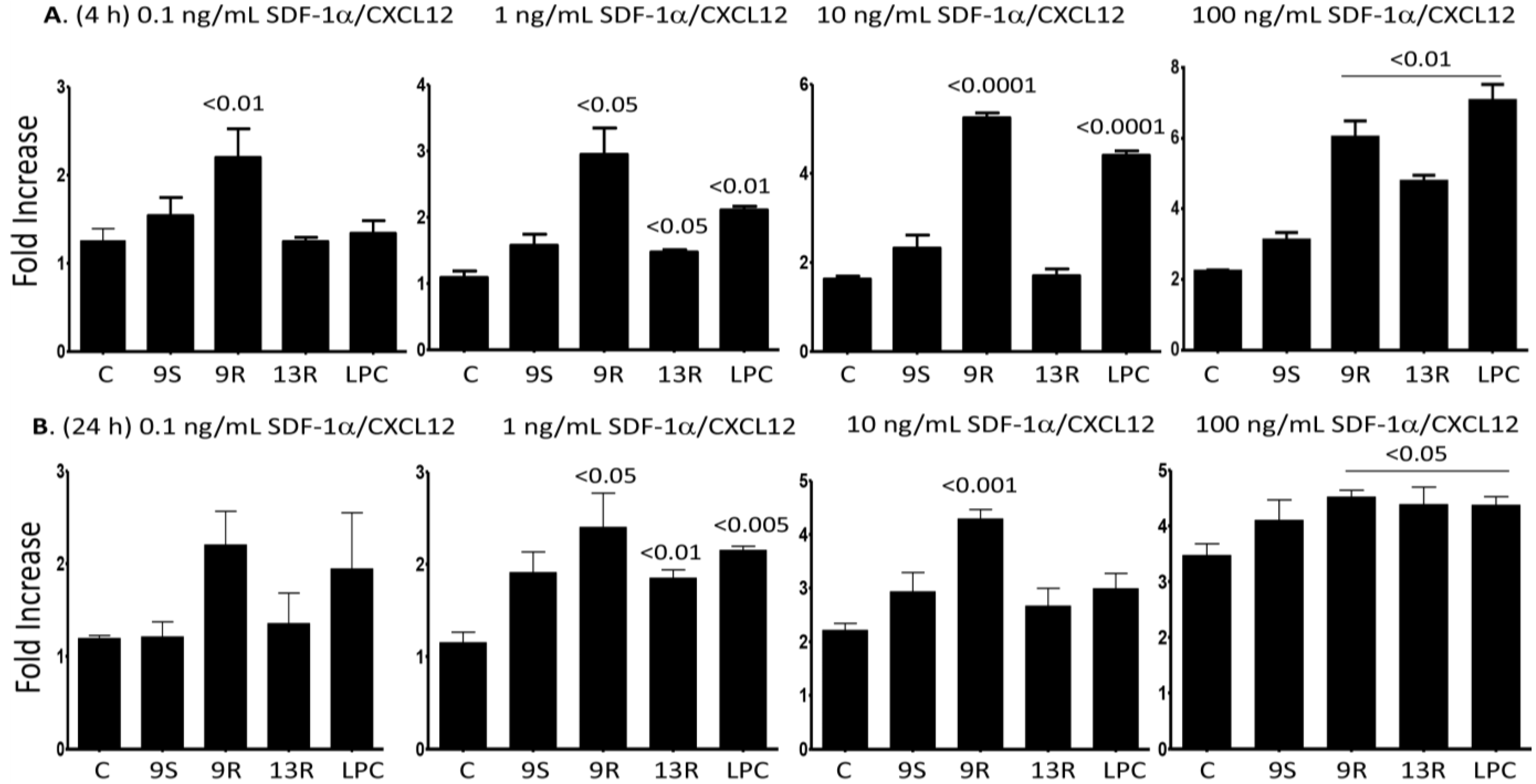
2.6. Oxidized Lipids and LPC Inhibit IL-6 Release from Monocytes
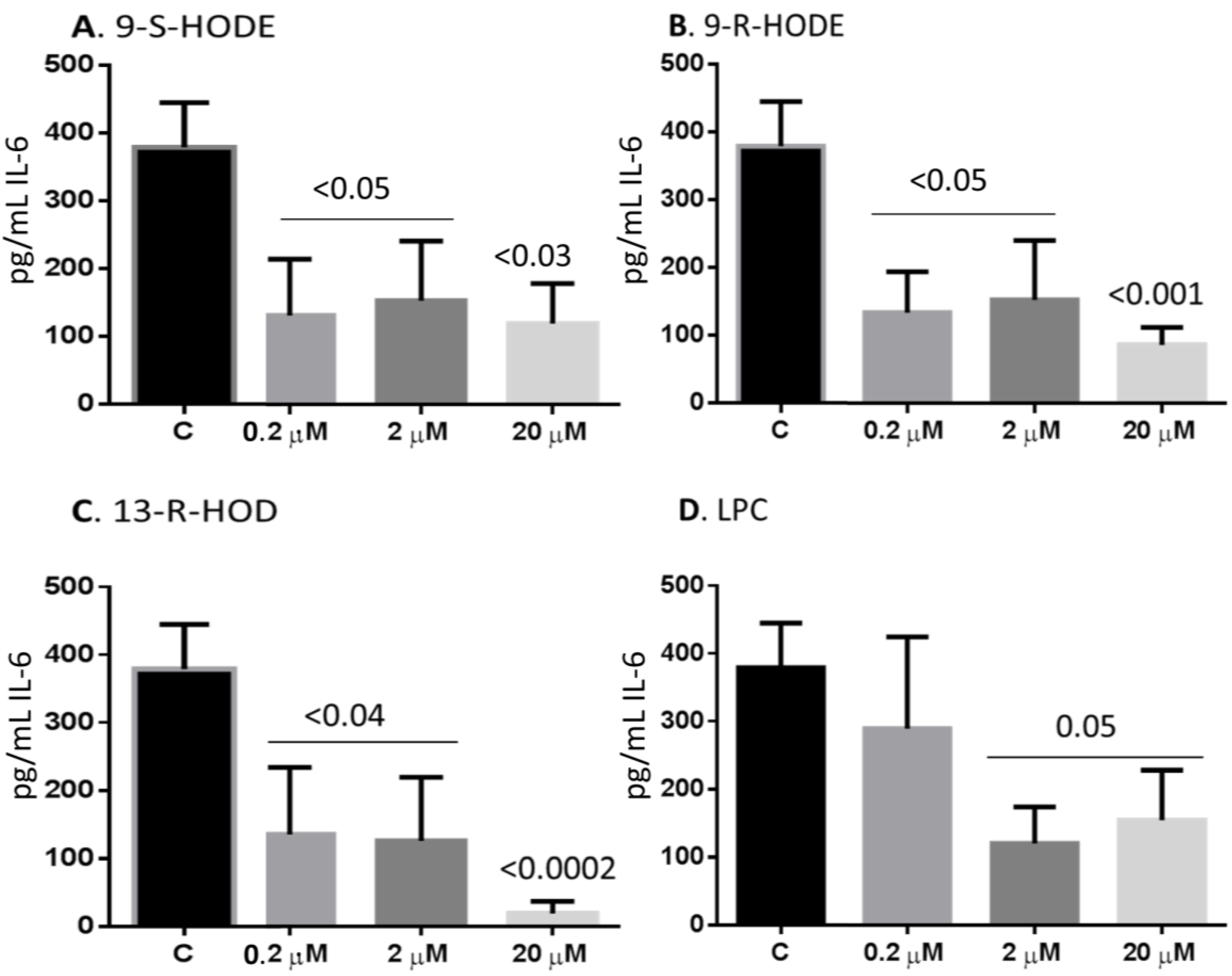
3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Preparation and Culture of Cells
4.3. In Vitro Chemotaxis Assay
4.4. Flow Cytometric Analysis
4.5. Mobilization of Intracellular Calcium
4.6. Detection of Cytokines and Chemokines Release Utilizing the ELISArray Kits
4.7. Detection of IL-6 Release by ELISA
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buja, L.M.; Nikolai, N. Anitschkow and the lipid hypothesis of atherosclerosis. Cardiovasc. Pathol. 2014, 23, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, J.A.; Martinez-Ruiz, A.; Sancho-Rodriguez, N.; Martinez-Hernandez, P.; Noguera-Velasco, J.A. The real role of prediagnostic high-density lipoprotein cholesterol and the cancer risk: A concise review. Eur. J. Clin. Invest. 2014, 44, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Jira, W.; Spiteller, G.; Carson, W.; Schramm, A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem. Phys. Lipids 1998, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H. Biosynthesis, metabolization and biological importance of the primary 15-lipoxygenase metabolites 15-hydro(pero)XY-5Z,8Z,11Z,13E-eicosatetraenoic acid and 13-hydro(pero)XY-9Z,11E-octadecadienoic acid. Prog. Lipid Res. 1996, 35, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E. Bio-Markers of lipid peroxidation in vivo: Hydroxyoctadecadienoic acid and hydroxycholesterol. Biofactors 2006, 27, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Izumi, T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat. 2009, 89, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-Independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chu, A.; Li, W.; Wang, B.; Shelton, F.; Otero, F.; Nguyen, D.G.; Caldwell, J.S.; Chen, Y.A. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem. 2009, 284, 12328–12338. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lee, Y.F.; Kim, E.; Chen, L.M.; Ni, J.; Fang, L.Y.; Liu, S.; Lin, S.J.; Abe, J.; Berk, B.; et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc. Natl. Acad. Sci. USA 2009, 106, 13353–13358. [Google Scholar] [CrossRef] [PubMed]
- Kveberg, L.; Bryceson, Y.; Inngjerdingen, M.; Rolstad, B.; Maghazachi, A.A. Sphingosine 1 phosphate induces the chemotaxis of human natural killer cells. Role for heterotrimeric G proteins and phosphoinositide 3 kinases. Eur. J. Immunol. 2002, 32, 1856–1864. [Google Scholar]
- Jin, Y.; Damaj, B.B.; Maghazachi, A.A. Human resting CD16-, CD16+ and IL-2-, IL-12-, IL-15- or IFN-alpha-activated natural killer cells differentially respond to sphingosylphosphorylcholine, lysophosphatidylcholine and platelet-activating factor. Eur. J. Immunol. 2005, 35, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Febbraio, M.; Sheibani, N.; Schmitt, D.; Silverstein, R.L.; Hajjar, D.P.; Cohen, P.A.; Frazier, W.A.; Hoff, H.F.; Hazen, S.L. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 2000, 105, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J. Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv. Exp. Med. Biol. 2012, 750, 2–13. [Google Scholar] [PubMed]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-Specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.L.; Binder, C.J.; Stockl, J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox. Signal. 2010, 12, 1009–1059. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B.; Murphy, R.C. New families of bioactive oxidized phospholipids generated by immune cells: Identification and signaling actions. Blood 2012, 120, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; de Assis, E.F.; Caiado, L.F.; Marathe, G.K.; Bozza, M.T.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M.; Bozza, P.T.; Castro-Faria-Neto, H.C. Monocyte chemoattractant protein-1 and 5-lipoxygenase products recruit leukocytes in response to platelet-activating factor-like lipids in oxidized low-density lipoprotein. J. Immunol. 2002, 168, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Jung, J.S.; Lee, J.E.; Lee, J.; Huh, S.O.; Kim, H.S.; Jung, K.C.; Cho, J.Y.; Nam, J.S.; Suh, H.W.; et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004, 10, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Rolin, J.; Maghazachi, A.A. Implications of chemokines, chemokine receptors, and inflammatory lipids in atherosclerosis. J. Leukoc. Biol. 2014, 95, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Benitez, S.; Camacho, M.; Arcelus, R.; Vila, L.; Bancells, C.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells. Relationship with electronegative LDL. Atherosclerosis 2004, 177, 299–305. [Google Scholar]
- Rolin, J.; Al-Jaderi, Z.; Maghazachi, A.A. Oxidized lipids and lysophosphatidylcholine induce the chemotaxis and intracellular calcium influx in natural killer cells. Immunobiology 2013, 218, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Welch, J.S.; Ricote, M.; Binder, C.J.; Willson, T.M.; Kelly, C.; Witztum, J.L.; Funk, C.D.; Conrad, D.; Glass, C.K. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 1999, 400, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Schild, R.L.; Schaiff, W.T.; Carlson, M.; Cronbach, E.J.; Nelson, D.M.; Sadovsky, Y. The activity of PPAR gamma in primary human trophoblasts is enhanced by oxidized lipids. J. Clin. Endocrinol. Metabolism 2002, 87, 1105–1110. [Google Scholar]
- Obinata, H.; Hattori, T.; Nakane, S.; Tatei, K.; Izumi, T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J. Biol. Chem. 2005, 280, 40676–40683. [Google Scholar] [CrossRef] [PubMed]
- Waddington, E.I.; Croft, K.D.; Sienuarine, K.; Latham, B.; Puddey, I.B. Fatty acid oxidation products in human atherosclerotic plaque: An analysis of clinical and histopathological correlates. Atherosclerosis 2003, 167, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Waddington, E.; Sienuarine, K.; Puddey, I.; Croft, K. Identification and quantitation of unique fatty acid oxidation products in human atherosclerotic plaque using high-performance liquid chromatography. Anal. Biochem. 2001, 292, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Witte, O.N.; Kabarowski, J.H.; Xu, Y.; Le, L.Q.; Zhu, K. Retraction. Science 2005, 307, 206. [Google Scholar] [CrossRef] [PubMed]
- Frasch, S.C.; Zemski-Berry, K.; Murphy, R.C.; Borregaard, N.; Henson, P.M.; Bratton, D.L. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J. Immunol. 2007, 178, 6540–6548. [Google Scholar] [CrossRef] [PubMed]
- Maghazachi, A.A. Intracellular signaling events at the leading edge of migrating cells. Int. J. Biochem. Cell Biol. 2000, 32, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Chang, M.K.; Boullier, A.; Green, S.R.; Li, A.; Glass, C.K.; Quehenberger, O. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J. Clin. Invest. 2000, 106, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Barlic, J.; Zhang, Y.; Foley, J.F.; Murphy, P.M. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor γ-dependent pathway. Circulation 2006, 114, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Zaguri, R.; Verbovetski, I.; Atallah, M.; Trahtemberg, U.; Krispin, A.; Nahari, E.; Leitersdorf, E.; Mevorach, D. Danger’ effect of low-density lipoprotein (LDL) and oxidized LDL on human immature dendritic cells. Clin. Exp. Immunol. 2007, 149, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Hong, K.H.; Ko, J.; Rhee, K.S.; Hong, M.K.; Kim, J.J.; Kim, Y.H.; Park, S.J. Lysophosphatidylcholine up-regulates CXCR4 chemokine receptor expression in human CD4 T cells. J. Leukoc. Biol. 2004, 76, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, G.; Tang, C.; Qiu, J.; Zhao, J.; Gregersen, H.; Deng, L. Upregulation of SDF-1 is associated with atherosclerosis lesions induced by LDL concentration polarization. Ann. Biomed. Eng. 2012, 40, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Schober, A.; Bot, I.; von Hundelshausen, P.; Liehn, E.A.; Mopps, B.; Mericskay, M.; Gierschik, P.; Biessen, E.A.; Weber, C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 2005, 96, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Abi-Younes, S.; Sauty, A.; Mach, F.; Sukhova, G.K.; Libby, P.; Luster, A.D. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ. Res. 2000, 86, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Kipps, T.J. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006, 107, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Stem Cell 2007, 1, 313–323. [Google Scholar]
- Stec, M.; Baran, J.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Gozdzik, J.; Siedlar, M.; Zembala, M. Chemokine receptors and chemokine production by CD34+ stem cell-derived monocytes in response to cancer cells. Anticancer Res. 2012, 32, 4749–4753. [Google Scholar] [PubMed]
- Schioppa, T.; Uranchimeg, B.; Saccani, A.; Biswas, S.K.; Doni, A.; Rapisarda, A.; Bernasconi, S.; Saccani, S.; Nebuloni, M.; Vago, L.; et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 2003, 198, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Machelon, V.; Coulomb-L’Hermin, A.; Borvak, J.; Nome, F.; Isaeva, T.; Wei, S.; Krzysiek, R.; Durand-Gasselin, I.; Gordon, A.; et al. Stromal-Derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat. Med. 2001, 7, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Linton, L.; Karlsson, M.; Grundstrom, J.; Hjalmarsson, E.; Lindberg, A.; Lindh, E.; Glise, H.; Befrits, R.; Janczewska, I.; Karlen, P.; et al. HLA-DR(hi) and CCR9 define a pro-inflammatory monocyte subset in IBD. Clin. Transl. Gastroenterol. 2012, 3, e29. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, C.; Cartwright, A.; Williams, H.; Haworth, O.; Williams, J.H.; Filer, A.; Salmon, M.; Buckley, C.D.; Middleton, J. Monocytes/macrophages express chemokine receptor CCR9 in rheumatoid arthritis and CCL25 stimulates their differentiation. Arthritis Res. Ther. 2010, 12, R161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Alla, J.; Langer, A.; Elzahwy, S.S.; Arman-Kalcek, G.; Streichert, T.; Quitterer, U. Angiotensin-Converting enzyme inhibition down-regulates the pro-atherogenic chemokine receptor 9 (CCR9)-chemokine ligand 25 (CCL25) axis. J. Biol. Chem. 2010, 285, 23496–23505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, B.; Schiller, J.; Wagner, U.; Hantzschel, H.; Arnold, K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: Investigations by 31P NMR and MALDI-TOF MS. Clin. Biochem. 2005, 38, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Muller, K.; Paasch, U.; Schiller, J. Lysophospholipids: Potential markers of diseases and infertility? Mini Rev. Med. Chem. 2012, 12, 74–86. [Google Scholar]
- Drobnik, W.; Liebisch, G.; Audebert, F.X.; Frohlich, D.; Gluck, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Spangelo, B.L.; Jarvis, W.D. Lysophosphatidylcholine stimulates interleukin-6 elease from rat anterior pituitary cells in vitro. Endocrinology 1996, 137, 4419–4426. [Google Scholar] [PubMed]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-Gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xia, M.; Zhu, H.; Wang, Q.; Li, Y.; Xiao, Y.; Zhao, T.; Tang, Z.; Ma, J.; Ling, W. Lysophosphatidylcholine promotes cholesterol efflux from mouse macrophage foam cells via PPARγ-LXRα-ABCA1-dependent pathway associated with apoE. Cell. Biochem. Funct. 2007, 25, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Czimmerer, Z.; Nagy, L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J. Allergy Clin. Immunol. 2013, 132, 264–286. [Google Scholar] [CrossRef] [PubMed]
- Oz-Arslan, D.; Ruscher, W.; Myrtek, D.; Ziemer, M.; Jin, Y.; Damaj, B.B.; Sorichter, S.; Idzko, M.; Norgauer, J.; Maghazachi, A.A. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J. Leukoc. Biol. 2006, 80, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Knudsen, E.; Wang, L.; Bryceson, Y.; Damaj, B.; Gessani, S.; Maghazachi, A.A. Sphingosine 1-phosphate is a novel inhibitor of T-cell proliferation. Blood 2003, 101, 4909–4915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghazachi, A.A.; Skalhegg, B.S.; Rolstad, B.; Al-Aoukaty, A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J. 1997, 11, 765–774. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rolin, J.; Vego, H.; Maghazachi, A.A. Oxidized Lipids and Lysophosphatidylcholine Induce the Chemotaxis, Up-Regulate the Expression of CCR9 and CXCR4 and Abrogate the Release of IL-6 in Human Monocytes. Toxins 2014, 6, 2840-2856. https://doi.org/10.3390/toxins6092840
Rolin J, Vego H, Maghazachi AA. Oxidized Lipids and Lysophosphatidylcholine Induce the Chemotaxis, Up-Regulate the Expression of CCR9 and CXCR4 and Abrogate the Release of IL-6 in Human Monocytes. Toxins. 2014; 6(9):2840-2856. https://doi.org/10.3390/toxins6092840
Chicago/Turabian StyleRolin, Johannes, Heidi Vego, and Azzam A. Maghazachi. 2014. "Oxidized Lipids and Lysophosphatidylcholine Induce the Chemotaxis, Up-Regulate the Expression of CCR9 and CXCR4 and Abrogate the Release of IL-6 in Human Monocytes" Toxins 6, no. 9: 2840-2856. https://doi.org/10.3390/toxins6092840



