Regulation of cry Gene Expression in Bacillus thuringiensis
Abstract
:1. Introduction
2. Transcriptional Regulation
| Gene | Sigma factors | Other TFs | References |
|---|---|---|---|
| cry1A | σE, σK | Spo0A (+); PDH E2 (+) | [7,8,9] |
| cry2A | σE | NA | [10] |
| cry2B | σE | NA | [10] |
| cry3A | σA | NA | [5] |
| cry4A | σE, σK, σH | PPK(+); Hpr/CcpA(−) | [11,12,13,14] |
| cry4B | σE, σK | NA | [11] |
| cry6Aa2 | NA | ORF2 (−) | [15] |
| cry8Ea1 | σE, σK, σH | NA | [16] |
| cry11A | σE, σK, σH | Spo0A (−) | [11] |
| cry15A | σE | NA | [17] |
| cry18A | σE, σK | NA | [18] |
2.1. Sporulation-Dependent cry Genes
2.2. Sporulation-Independent cry Genes
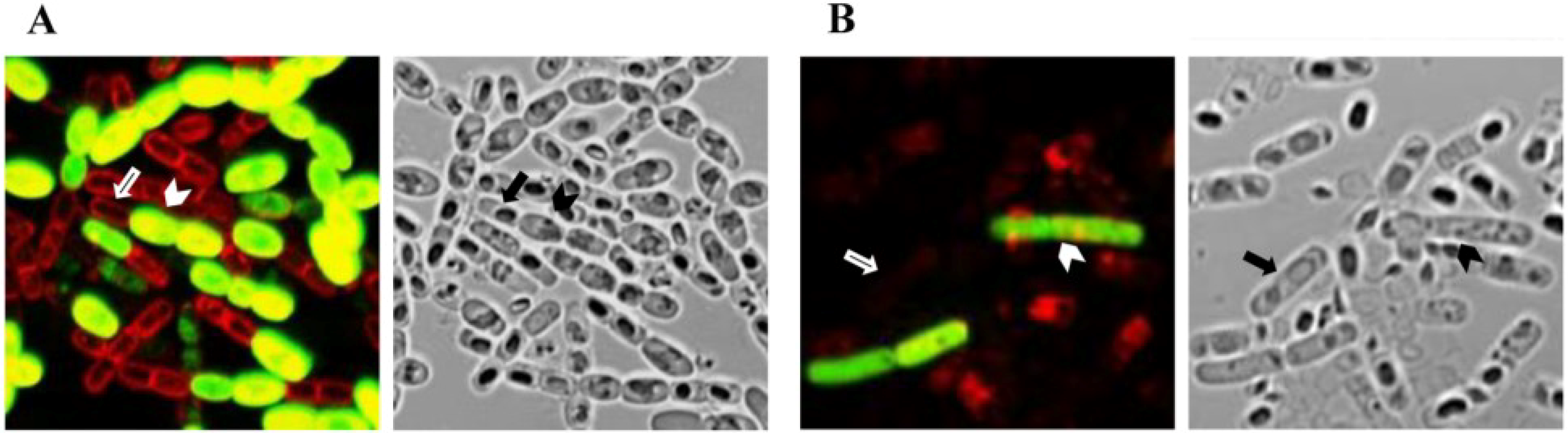
2.3. Regulation of cry Gene Expression by Other Factors
2.3.1. The Transcriptional Regulator Spo0A
2.3.2. The E2 Subunit of the Pyruvate Dehygrogenase (PDH) Complex
2.3.3. Regulation by Two Negative Factors
3. mRNA Stability
3.1. 3’ Terminal Structure
3.2. 5’ mRNA Stabilizer

4. Metabolic Regulation of Cry Protein Production
4.1. Regulation by the Sigma 54 Factor
4.2. Polyphosphate Kinase (PPK) and Polyphosphate Metabolism
4.3. Catabolic Repression by Glucose
5. Crystallization of the Cry Proteins
6. Patterns of Crystal Production
7. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Vilas-Boas, G.T.; Peruca, A.P.; Arantes, O.M. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can. J. Microbiol. 2007, 53, 673–687. [Google Scholar] [CrossRef]
- Kolstø, A.B.; Lereclus, D.; Mock, M. Genome structure and evolution of the Bacillus cereus group. Curr Top. Microbiol. Immunol. 2002, 264, 95–108. [Google Scholar]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect. Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant. Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J. Bacteriol. 1995, 177, 6027–6032. [Google Scholar]
- Baum, J.A.; Malvar, T. Regulation of insecticidal crystal protein production in Bacillus thuringiensis. Mol. Microbiol. 1995, 18, 1–12. [Google Scholar]
- Yang, H.; Wang, P.; Peng, Q.; Rong, R.; Liu, C.; Lereclus, D.; Zhang, J.; Song, F.; Huang, D. Weak transcription of the cry1Ac gene in nonsporulating Bacillus thuringiensis cells. Appl. Environ. Microbiol. 2012, 78, 6466–6474. [Google Scholar] [CrossRef]
- Perez-Garcia, G.; Basurto-Rios, R.; Ibarra, J.E. Potential effect of a putative sigma(H)-driven promoter on the over expression of the Cry1Ac toxin of Bacillus thuringiensis. J. Invertebr. Pathol. 2010, 104, 140–146. [Google Scholar] [CrossRef]
- Walter, T.; Aronson, A. Specific binding of the E2 subunit of pyruvate dehydrogenase to the upstream region of Bacillus thuringiensis protoxin genes. J. Biol. Chem. 1999, 274, 7901–7906. [Google Scholar] [CrossRef]
- Widner, W.R.; Whiteley, H.R. Two highly related insecticidal crystal proteins of Bacillus thuringiensis subsp. kurstaki possess different host range specificities. J. Bacteriol. 1989, 171, 965–974. [Google Scholar]
- Poncet, S.; Dervyn, E.; Klier, A.; Rapoport, G. Spo0A represses transcription of the cry toxin genes in Bacillus thuringiensis. Microbiology 1997, 143, 2743–2751. [Google Scholar] [CrossRef]
- Doruk, T.; Avican, U.; Camci, I.Y.; Gedik, S.T. Overexpression of polyphosphate kinase gene (ppk) increases bioinsecticide production by Bacillus thuringiensis. Microbiol. Res. 2013, 168, 199–203. [Google Scholar] [CrossRef]
- Khan, S.R.; Banerjee-Bhatnagar, N. Loss of catabolite repression function of HPr, the phosphocarrier protein of the bacterial phosphotransferase system, affects expression of the cry4A toxin gene in Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 2002, 184, 5410–5417. [Google Scholar] [CrossRef]
- Kant, S.; Kapoor, R.; Banerjee, N. Identification of a catabolite-responsive element necessary for regulation of the cry4A gene of Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 2009, 191, 4687–4692. [Google Scholar] [CrossRef]
- Yu, Z.; Bai, P.; Ye, W.; Zhang, F.; Ruan, L.; Sun, M. A novel negative regulatory factor for nematicidal Cry protein gene expression in Bacillus thuringiensis. J. Microbiol. Biotechnol. 2008, 18, 1033–1039. [Google Scholar]
- Du, L.; Qiu, L.; Peng, Q.; Lereclus, D.; Zhang, J.; Song, F.; Huang, D. Identification of the promoter in the intergenic region between orf1 and cry8Ea1 controlled by sigma H factor. Appl. Environ. Microbiol. 2012, 78, 4164–4168. [Google Scholar]
- Brown, K.L. Transcriptional regulation of the Bacillus thuringiensis subsp. thompsoni crystal protein gene operon. J. Bacteriol. 1993, 175, 7951–7957. [Google Scholar]
- Zhang, J.; Schairer, H.U.; Schnetter, W.; Lereclus, D.; Agaisse, H. Bacillus popilliae cry18Aa operon is transcribed by sigmaE and sigmaK forms of RNA polymerase from a single initiation site. Nucl. Acids Res. 1998, 26, 1288–1293. [Google Scholar] [CrossRef]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 2004, 7, 579–586. [Google Scholar] [CrossRef]
- Lereclus, D.; Agaisse, H. Toxin and virulence gene expression in Bacillus thuringiensis. In Entomopathogenic Bacteria: From Laboratory to Field Application; Charles, J.F., Delécluse, A., Nielsen-Leroux, C., Eds.; Kluwer Academic Publishers: Heidelberg, Germany, 2000; pp. 127–142. [Google Scholar]
- Aronson, A. Sporulation and delta-endotoxin synthesis by Bacillus thuringiensis. Cell. Mol. Life Sci. 2002, 59, 417–425. [Google Scholar]
- Wang, J.; Mei, H.; Qian, H.; Tang, Q.; Liu, X.; Yu, Z.; He, J. Expression profile and regulation of spore and parasporal crystal formation-associated genes in Bacillus thuringiensis. J. Proteome Res. 2013, 12, 5487–5501. [Google Scholar] [CrossRef]
- Bravo, A.; Agaisse, H.; Salamitou, S.; Lereclus, D. Analysis of cryIAa expression in sigE and sigK mutants of Bacillus thuringiensis. Mol. Gen. Genet. 1996, 250, 734–741. [Google Scholar]
- Yoshisue, H.; Ihara, K.; Nishimoto, T.; Sakai, H.; Komano, T. Expression of the genes for insecticidal crystal proteins in Bacillus thuringiensis: CryIVA, not cryIVB, is transcribed by RNA polymerase containing sigma H and that containing sigma E. FEMS Microbiol. Lett. 1995, 127, 65–72. [Google Scholar]
- Dervyn, E.; Poncet, S.; Klier, A.; Rapoport, G. Transcriptional regulation of the cryIVD gene operon from Bacillus thuringiensis subsp. Israelensis. J. Bacteriol. 1995, 177, 2283–2291. [Google Scholar]
- Agaisse, H.; Lereclus, D. Expression in Bacillus subtilis of the Bacillus thuringiensis cryIIIA toxin gene is not dependent on a sporulation-specific sigma factor and is increased in a spo0A mutant. J. Bacteriol. 1994, 176, 4734–4741. [Google Scholar]
- Malvar, T.; Baum, J. Tn5401 disruption of the spo0F gene, identified by direct chromosomal sequencing, results in cryIIIA overproduction in Bacillus thuringiensis. J. Bacteriol. 1994, 176, 4750–4753. [Google Scholar]
- Lereclus, D.; Agaisse, H.; Gominet, M.; Chaufaux, J. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo0A mutant. Nat. Biotechnol. 1995, 13, 67–71. [Google Scholar]
- Deng, C.; Slamti, L.; Raymond, B.; Liu, G.; Lemy, C.; Gominet, M.; Yang, J.; Wang, H.; Peng, Q.; Zhang, J.; et al. Division of labour and terminal differentiation in a novel Bacillus thuringiensis strain. ISME J. 2014, in press. [Google Scholar]
- Molle, V.; Fujita, M.; Jensen, S.T.; Eichenberger, P.; Gonzalez-Pastor, J.E.; Liu, J.S.; Losick, R. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 2003, 50, 1683–1701. [Google Scholar] [CrossRef]
- Berg, A.; de Kok, A. 2-Oxo acid dehydrogenase multienzyme complexes. The central role of the lipoyl domain. Biol. Chem. 1997, 378, 617–634. [Google Scholar]
- Gao, H.; Jiang, X.; Pogliano, K.; Aronson, A.I. The E1beta and E2 subunits of the Bacillus subtilis pyruvate dehydrogenase complex are involved in regulation of sporulation. J. Bacteriol. 2002, 184, 2780–2788. [Google Scholar] [CrossRef]
- Glatron, M.F.; Rapoport, G. Biosynthesis of the parasporal inclusion of Bacillus thuringiensis: Half-life of its corresponding messenger RNA. Biochimie 1972, 54, 1291–1301. [Google Scholar] [CrossRef]
- Wong, H.C.; Chang, S. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc. Natl. Acad Sci. USA 1986, 83, 3233–3237. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. Structural and functional analysis of the promoter region involved in full expression of the cryIIIA toxin gene of Bacillus thuringiensis. Mol. Microbiol. 1994, 13, 97–107. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. STAB-SD: A Shine-Dalgarno sequence in the 5’ untranslated region is a determinant of mRNA stability. Mol. Microbiol. 1996, 20, 633–643. [Google Scholar]
- Mathy, N.; Benard, L.; Pellegrini, O.; Daou, R.; Wen, T.; Condon, C. 5’-to-3’ exoribonuclease activity in bacteria: Role of RNase J1 in rRNA maturation and 5’ stability of mRNA. Cell 2007, 129, 681–692. [Google Scholar]
- Wang, J.; Mei, H.; Zheng, C.; Qian, H.; Cui, C.; Fu, Y.; Su, J.; Liu, Z.; Yu, Z.; He, J. The metabolic regulation of sporulation and parasporal crystal formation in Bacillus thuringiensis revealed by transcriptomics and proteomics. Mol. Cell. Proteomics 2013, 12, 1363–1376. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, Q.; Song, F.; Jiang, Y.; Sun, C.; Zhang, J.; Huang, D. Structure and regulation of the gab gene cluster, involved in the gamma-aminobutyric acid shunt, are controlled by a sigma 54 factor in Bacillus thuringiensis. J. Bacteriol. 2010, 192, 346–355. [Google Scholar]
- Aronson, J.N.; Borris, D.P.; Doerner, J.F.; Akers, E. Gamma-aminobutyric acid pathway and modified tricarboxylic acid cycle activity during growth and sporulation of Bacillus thuringiensis. Appl. Microbiol. 1975, 30, 489–492. [Google Scholar]
- Ali, N.O.; Bignon, J.; Rapoport, G.; Débarbouillé, M. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J. Bacteriol. 2001, 183, 2497–2504. [Google Scholar]
- Kurt, A.; Ozkan, M.; Ozcengiz, G. Inorganic phosphate has a crucial effect on Cry3Aa delta-endotoxin production. Lett. Appl. Microbiol. 2005, 41, 303–308. [Google Scholar]
- Ozkan, M.; Dilek, F.B.; Yetis, U.; Ozcengiz, G. Nutritional and cultural parameters influencing antidipteran delta-endotoxin production. Res. Microbiol. 2003, 154, 49–53. [Google Scholar]
- Banerjee-Bhatnagar, N. Inorganic phosphate regulates CryIVA protoxin expression in Bacillus thuringiensis israelensis. Biochem. Biophys. Res. Commun. 1999, 262, 359–364. [Google Scholar] [CrossRef]
- Ahn, K.; Kornberg, A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J. Biol. Chem. 1990, 265, 11734–11739. [Google Scholar]
- Akiyama, M.; Crooke, E.; Kornberg, A. An exopolyphosphatase of Escherichia coli. The enzyme and its ppx gene in a polyphosphate operon. J. Biol. Chem. 1993, 268, 633–639. [Google Scholar]
- Fujita, Y.; Miwa, Y.; Galinier, A.; Deutscher, J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol. Microbiol. 1995, 17, 953–960. [Google Scholar]
- Jones, B.E.; Dossonnet, V.; Kuster, E.; Hillen, W.; Deutscher, J.; Klevit, R.E. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J. Biol. Chem. 1997, 272, 26530–26535. [Google Scholar]
- Hueck, C.J.; Hillen, W. Catabolite repression in Bacillus subtilis: A global regulatory mechanism for the gram-positive bacteria? Mol. Microbiol. 1995, 15, 395–401. [Google Scholar] [CrossRef]
- Banerjee-Bhatnagar, N. Modulation of Cry IV A toxin protein expression by glucose in Bacillus thuringiensis israelensis. Biochem. Biophys. Res. Commun. 1998, 252, 402–406. [Google Scholar] [CrossRef]
- Barboza-Corona, J.E.; Park, H.W.; Bideshi, D.K.; Federici, B.A. The 60-kilodalton protein encoded by orf2 in the cry19A operon of Bacillus thuringiensis subsp. jegathesan functions like a C-terminal crystallization domain. Appl. Environ. Microbiol. 2012, 78, 2005–2012. [Google Scholar]
- Ge, B.; Bideshi, D.; Moar, W.J.; Federici, B.A. Differential effects of helper proteins encoded by the cry2A and cry11A operons on the formation of Cry2A inclusions in Bacillus thuringiensis. FEMS Microbiol. Lett. 1998, 165, 35–41. [Google Scholar] [CrossRef]
- Staples, N.; Ellar, D.; Crickmore, N. Cellular localization and characterization of the Bacillus thuringiensis Orf2 crystallization factor. Curr. Microbiol. 2001, 42, 388–392. [Google Scholar] [CrossRef]
- De Maagd, R.A.; Bravo, A.; Berry, C.; Crickmore, N.; Schnepf, H.E. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 2003, 37, 409–433. [Google Scholar]
- Wu, D.; Federici, B.A. A 20-kilodalton protein preserves cell viability and promotes CytA crystal formation during sporulation in Bacillus thuringiensis. J. Bacteriol. 1993, 175, 5276–5280. [Google Scholar]
- Shao, Z.; Liu, Z.; Yu, Z. Effects of the 20-kilodalton helper protein on Cry1Ac production and spore formation in Bacillus thuringiensis. Appl. Environ. Microbiol. 2001, 67, 5362–5369. [Google Scholar] [CrossRef]
- Yoshisue, H.; Yoshida, K.; Sen, K.; Sakai, H.; Komano, T. Effects of Bacillus thuringiensis var. israelensis 20-kDa protein on production of the Bti 130-kDa crystal protein in Escherichia coli. Biosci. Biotechnol. Biochem. 1992, 56, 1429–1433. [Google Scholar]
- Wu, D.; Federici, B.A. Improved production of the insecticidal CryIVD protein in Bacillus thuringiensis using cryIA(c) promoters to express the gene for an associated 20-kDa protein. Appl. Microbiol. Biotechnol. 1995, 42, 697–702. [Google Scholar]
- Park, H.W.; Bideshi, D.K.; Federici, B.A. Molecular genetic manipulation of truncated Cry1C protein synthesis in Bacillus thuringiensis to improve stability and yield. Appl. Environ. Microbiol. 2000, 66, 4449–4455. [Google Scholar] [CrossRef]
- Rang, C.; Bes, M.; Lullien-Pellerin, V.; Wu, D.; Federici, B.A.; Frutos, R. Influence of the 20-kDa protein from Bacillus thuringiensis ssp. israelensis on the rate of production of truncated Cry1C proteins. FEMS Microbiol. Lett. 1996, 141, 261–264. [Google Scholar] [CrossRef]
- Shi, Y.X.; Yuan, M.J.; Chen, J.W.; Sun, F.; Pang, Y. Effects of helper protein P20 from Bacillus thuringiensis on Vip3A expression. Acta Microbiol. Sinica 2006, 46, 85–89. [Google Scholar]
- Park, H.W.; Bideshi, D.K.; Federici, B.A. The 20-kDa protein of Bacillus thuringiensis subsp. israelensis enhances Bacillus sphaericus 2362 bin toxin synthesis. Curr. Microbiol. 2007, 55, 119–124. [Google Scholar] [CrossRef]
- McLean, K.M.; Whiteley, H.R. Expression in Escherichia coli of a cloned crystal protein gene of Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 1987, 169, 1017–1023. [Google Scholar]
- Adams, L.F.; Visick, J.E.; Whiteley, H.R. A 20-kilodalton protein is required for efficient production of the Bacillus thuringiensis subsp. israelensis 27-kilodalton crystal protein in Escherichia coli. J. Bacteriol. 1989, 171, 521–530. [Google Scholar]
- Visick, J.E.; Whiteley, H.R. Effect of a 20-kilodalton protein from Bacillus thuringiensis subsp. israelensis on production of the CytA protein by Escherichia coli. J. Bacteriol. 1991, 173, 1748–1756. [Google Scholar]
- Zhu, Y.; Ji, F.; Shang, H.; Zhu, Q.; Wang, P.; Xu, C.; Deng, Y.; Peng, D.; Ruan, L.; Sun, M. Gene clusters located on two large plasmids determine spore crystal association (SCA) in Bacillus thuringiensis subsp. finitimus strain YBT-020. PLoS One 2011, 6, e27164. [Google Scholar]
- Debro, L.; Fitz-James, P.C.; Aronson, A. Two different parasporal inclusions are produced by Bacillus thuringiensis subsp. finitimus. J. Bacteriol. 1986, 165, 258–268. [Google Scholar]
- Arantes, O.; Lereclus, D. Construction of cloning vectors for Bacillus thuringiensis. Gene 1991, 108, 115–119. [Google Scholar] [CrossRef]
- Sanchis, V.; Agaisse, H.; Chaufaux, J.; Lereclus, D. A recombinase-mediated system for elimination of antibiotic resistance gene markers from gennetically engineered Bacillus thuringiensis strains. Appl. Environ. Microbiol. 1997, 63, 779–784. [Google Scholar]
- Hernandez-Soto, A.; Del Rincon-Castro, M.C.; Espinoza, A.M.; Ibarra, J.E. Parasporal body formation via overexpression of the Cry10Aa toxin of Bacillus thuringiensis subsp. israelensis, and Cry10Aa-Cyt1Aa synergism. Appl. Environ. Microbiol. 2009, 75, 4661–4667. [Google Scholar]
- Park, H.W.; Ge, B.; Bauer, L.S.; Federici, B.A. Optimization of Cry3A yields in Bacillus thuringiensis by use of sporulation-dependent promoters in combination with the STAB-SD mRNA sequence. Appl. Environ. Microbiol. 1998, 64, 3932–3938. [Google Scholar]
- Sanchis, V.; Agaisse, H.; Chaufaux, J.; Lereclus, D. Construction of new insecticidal Bacillus thuringiensis recombinant strains by using the sporulation non-dependent expression system of cryIIIA and a site specific recombination vector. J. Biotechnol. 1996, 48, 81–96. [Google Scholar] [CrossRef]
- Sanchis, V.; Gohar, M.; Chaufaux, J.; Arantes, O.; Meier, A.; Agaisse, H.; Cayley, J.; Lereclus, D. Development and field performance of a broad-spectrum nonviable asporogenic recombinant strain of Bacillus thuringiensis with greater potency and UV resistance. Appl. Environ. Microbiol. 1999, 65, 4032–4039. [Google Scholar]
- Lereclus, D.; Vallade, M.; Chaufaux, J.; Arantes, O.; Rambaud, S. Expansion of insecticidal host range of Bacillus thuringiensis by in vivo genetic recombination. Nat. Biotechnol. 1992, 10, 418–421. [Google Scholar] [CrossRef]
- Yan, G.; Song, F.; Shu, C.; Liu, J.; Liu, C.; Huang, D.; Feng, S.; Zhang, J. An engineered Bacillus thuringiensis strain with insecticidal activity against Scarabaeidae (Anomala corpulenta) and Chrysomelidae (Leptinotarsa decemlineata and Colaphellus bowringi). Biotechnol. Lett. 2009, 31, 697–703. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Song, F.; Wu, J.; Feng, S.; Huang, D. Engineered Bacillus thuringiensis GO33A with broad insecticidal activity against lepidopteran and coleopteran pests. Appl. Microbiol. Biotechnol. 2006, 72, 924–930. [Google Scholar] [CrossRef]
- Song, R.; Peng, D.; Yu, Z.; Sun, M. Carboxy-terminal half of Cry1C can help vegetative insecticidal protein to form inclusion bodies in the mother cell of Bacillus thuringiensis. Appl. Microbiol. Biotechnol. 2008, 80, 647–654. [Google Scholar]
- Raymond, B.; West, S.A.; Griffin, A.S.; Bonsall, M.B. The dynamics of cooperative bacterial virulence in the field. Science 2012, 337, 85–88. [Google Scholar] [CrossRef]
- Raymond, B.; Bonsall, M.B. Cooperation and the evolutionary ecology of bacterial virulence: The Bacillus cereus group as a novel study system. BioEssays 2013, 35, 706–716. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deng, C.; Peng, Q.; Song, F.; Lereclus, D. Regulation of cry Gene Expression in Bacillus thuringiensis. Toxins 2014, 6, 2194-2209. https://doi.org/10.3390/toxins6072194
Deng C, Peng Q, Song F, Lereclus D. Regulation of cry Gene Expression in Bacillus thuringiensis. Toxins. 2014; 6(7):2194-2209. https://doi.org/10.3390/toxins6072194
Chicago/Turabian StyleDeng, Chao, Qi Peng, Fuping Song, and Didier Lereclus. 2014. "Regulation of cry Gene Expression in Bacillus thuringiensis" Toxins 6, no. 7: 2194-2209. https://doi.org/10.3390/toxins6072194




