Toxicity of Parasporin-4 and Health Effects of Pro-parasporin-4 Diet in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acute Toxicity of PS4 for ICR Mice
| Dose amount (μg) | Number of administrations | Number of deaths | Mortality (%) |
|---|---|---|---|
| 3 | 5 | 0 | 0 |
| 6 | 9 | 7 | 78 |
| 12 | 9 | 8 | 89 |
| 24 | 5 | 5 | 100 |
| 48 | 4 | 4 | 100 |
2.2. Activation of ProPS4 in the Digestive Tract of Mouse
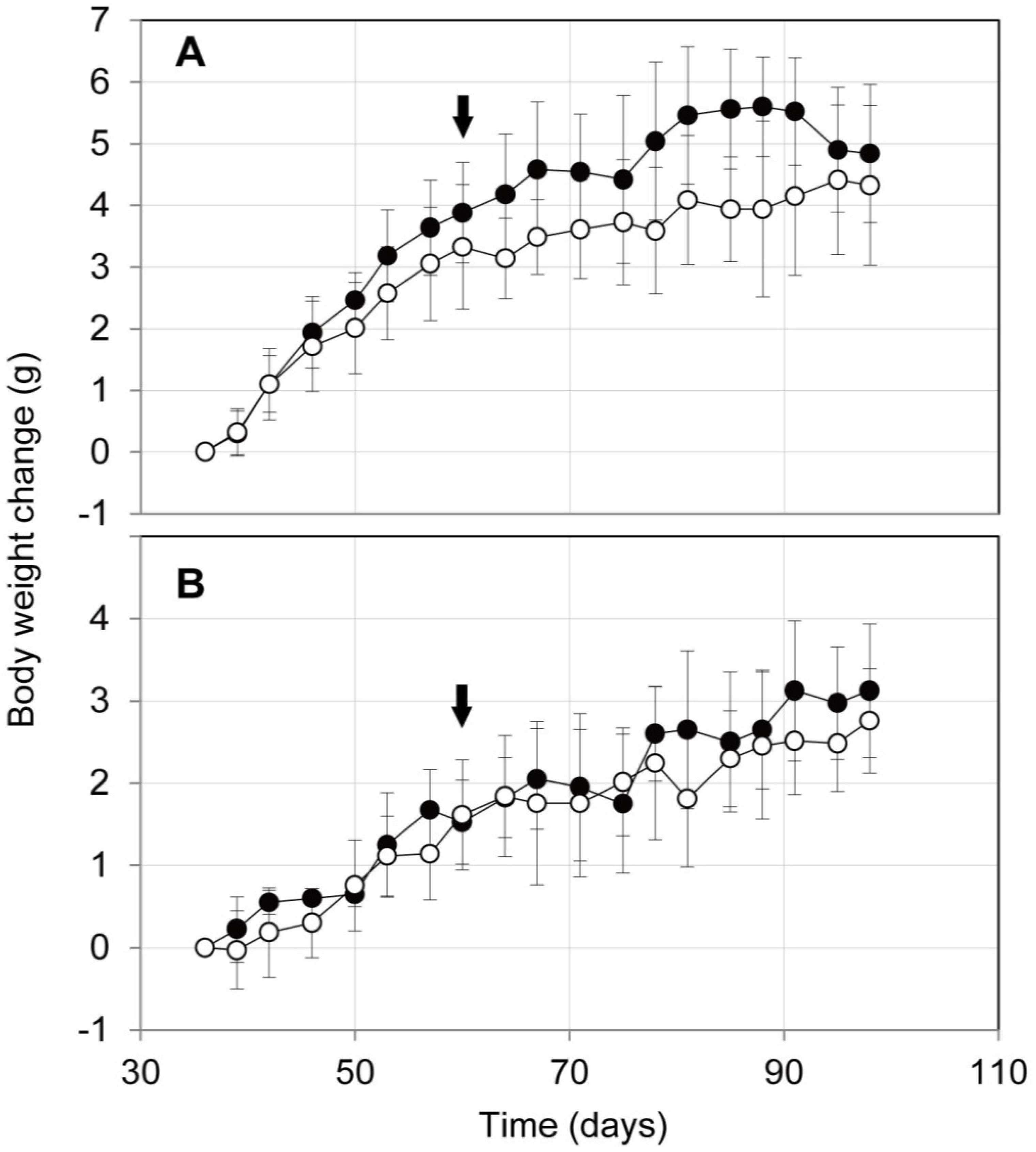
2.3. Influence on Mouse Health of the Oral Administration of ProPS4
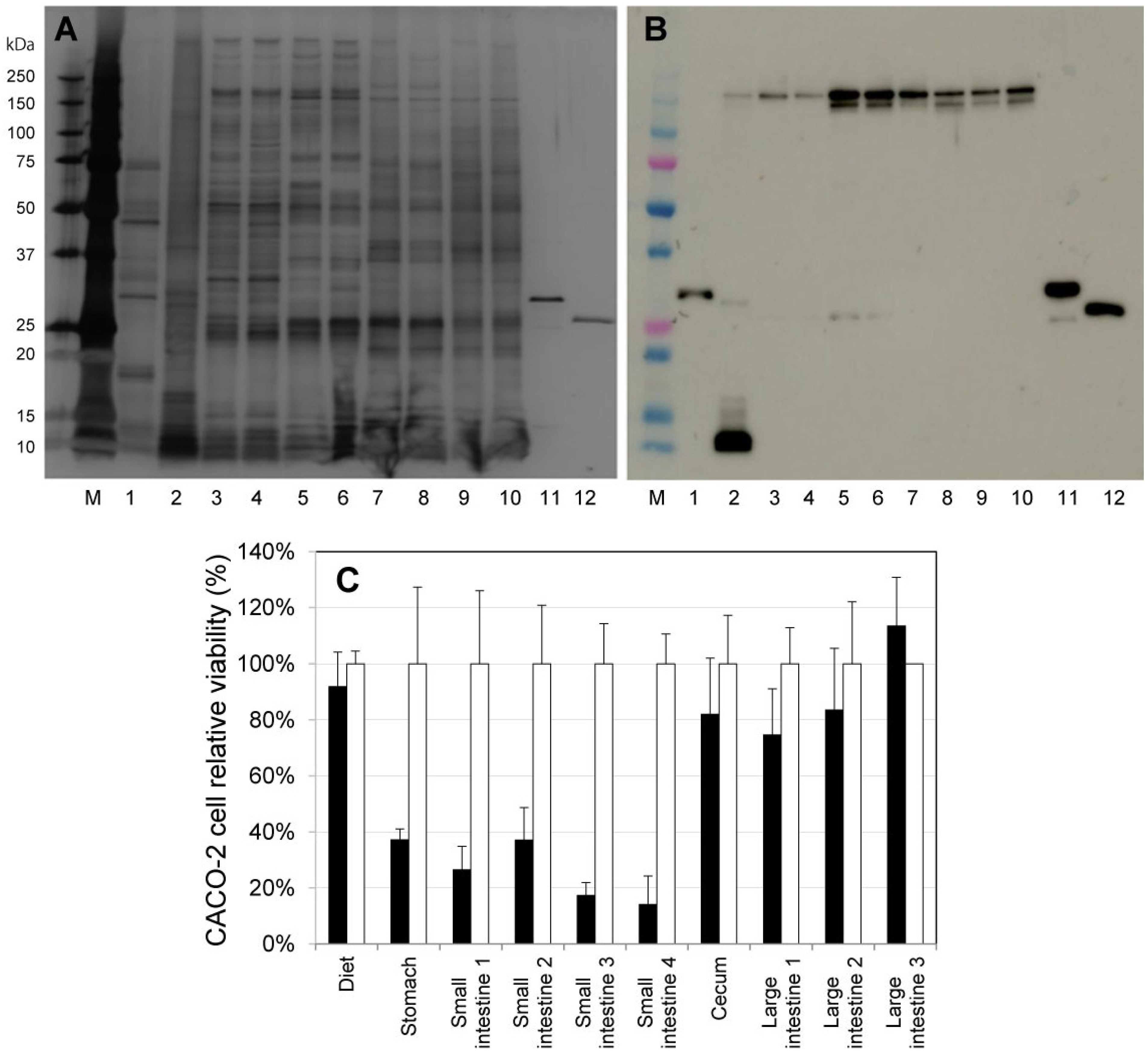
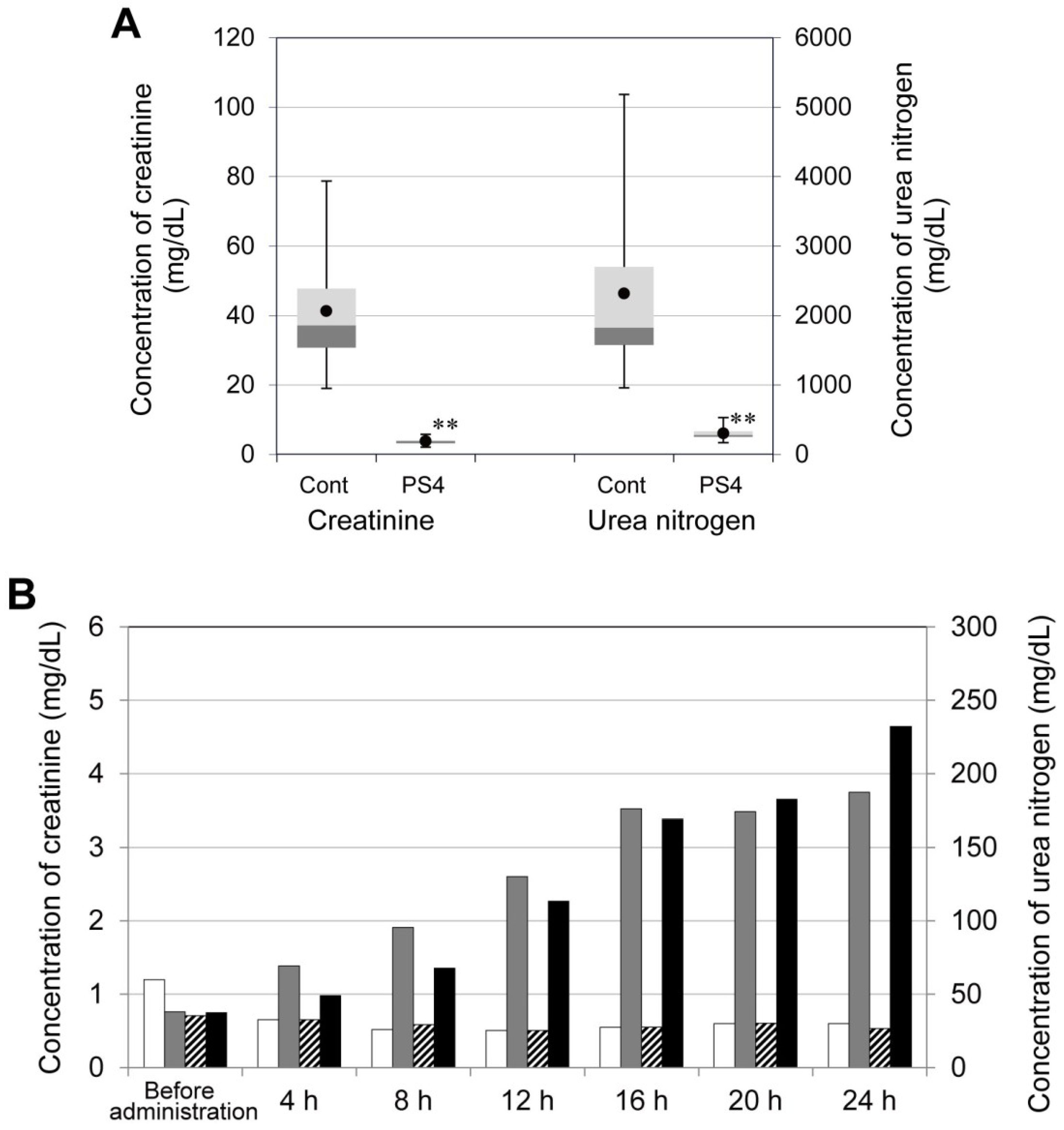
2.4. Influence of PS4 Injection on Kidney


3. Experimental Section
3.1. Preparation of Inclusion Body of Recombinant ProPS4 and Purification of PS4
3.2. Animals
3.3. Acute Toxicity of PS4 in ICR Mice
3.4. Oral Administration of ProPS4 and Activation of the Toxin in the Digestive Tract
3.5. Body Weight Changes of Mice after Oral Administration of ProPS4
3.6. Concentrations of Cations, Creatinine, and Urea Nitrogen in Urine after Administration of PS4
3.7. Concentration of Creatinine and Urea Nitrogen in Mouse Serum after Administration of PS4
3.8. Preparation of Kidney Slices of Mice Administered with PS4
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mizuki, E.; Park, Y.-S.; Saitoh, H.; Yamashita, S.; Akao, T.; Higuchi, K.; Ohba, M. Parasporin, a human leukemic cell-recognizing parasporal protein of Bacillus thuringiensis. Clin. Diagn. Lab. Immunol. 2000, 7, 625–634. [Google Scholar]
- Beegle, C.C.; Yamamoto, T. History of Bacillus thuringiensis Berliner research and development. Can. Entomol. 1992, 124, 587–616. [Google Scholar] [CrossRef]
- Schnepf, E.; Crickmore, N.; van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar]
- Ohba, M. Bacillus thuringiensis populations naturally occurring on mulberry leaves: A possible source of the populations associated with silkworm-rearing insectaries. J. Appl. Microbiol. 1996, 80, 56–64. [Google Scholar]
- Ohba, M.; Mizuki, E.; Crickmore, N.; Coté, J.-C.; Nagamatsu, Y.; Kitada, S.; Sakai, H.; Harata, K.; Shin, T.; Okumura, S. Parasporin Nomenclature. Available online: http://parasporin.fitc.pref.fukuoka.jp/ (accessed on 14 July 2014).
- Poornima, K.; Saranya, V.; Abirami, P.; Binuramesh, C.; Suguna, P.; Selvanayagam, P.; Shenbagarathai, R. Phenotypic and genotypic characterization of Bt LDC-391 strain that produce cytocidal proteins against human cancer cells. Bioinformation 2012, 8, 461–465. [Google Scholar] [CrossRef]
- Gonzalez, E.; Granados, J.C.; Short, J.D.; Ammons, D.R.; Rampersad, J. Parasporins from a Caribbean Island: Evidence for a globally dispersed Bacillus thuringiensis strain. Curr. Microbiol. 2011, 62, 1643–1648. [Google Scholar] [CrossRef]
- Al-Sayes, F.; Azhar, F.; Hindawi, S. Antimalignancy activity of Bacillus thuringiensis serovar dakota (H15) in vivo. World J. Med. Sci. 2011, 6, 6–16. [Google Scholar]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Inouye, K.; Mizuki, E. Mode of action of parasporin-4, a cytocidal protein from Bacillus thuringiensis. Biochim. Biophys. Acta 2011, 1808, 1476–1482. [Google Scholar]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Wasano, N.; Yamashita, S.; Kusumoto, K.; Akao, T.; Mizuki, E.; Ohba, M.; Inouye, K. Identification of a novel cytotoxic protein, Cry45Aa, from Bacillus thuringiensis A1470 and its selective cytotoxic activity against various mammalian cell lines. J. Agric. Food Chem. 2005, 53, 6313–6318. [Google Scholar] [CrossRef]
- Okumura, S.; Ishikawa, T.; Saitoh, H.; Akao, T.; Mizuki, E. Identification of a second cytotoxic protein produced by Bacillus thuringiensis A1470. Biotechnol. Lett. 2013, 35, 1889–1894. [Google Scholar] [CrossRef]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Mizuki, E.; Inouye, K. Identification and characterization of a novel cytotoxic protein, parasporin-4, produced by Bacillus thuringiensis A1470 strain. Biotechnol. Annu. Rev. 2008, 14, 225–252. [Google Scholar] [CrossRef]
- Okumura, S.; Saitoh, H.; Wasano, N.; Katayama, H.; Higuchi, K.; Mizuki, E.; Inouye, K. Efficient solubilization, activation, and purification of recombinant Cry45Aa of Bacillus thuringiensis expressed as inclusion bodies in Escherichia coli. Protein Expr. Purif. 2006, 47, 144–151. [Google Scholar]
- Höfte, H.; Whiteley, H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar]
- Fackrell, H.B.; Wiseman, G.M. Properties of the gamma haemolysin of Staphylococcus aureus “Smith 5R”. J. Gen. Microbiol. 1976, 92, 11–24. [Google Scholar] [CrossRef]
- Schein, C.H. Solubility as a function of protein structure and solvent components. Biotechnology 1990, 8, 308–317. [Google Scholar] [CrossRef]
- Oneda, H.; Inouye, K. Refolding and recovery of recombinant human matrix metalloproteinase 7 (matrilysin) from inclusion bodies expressed by Escherichia coli. J. Biochem. (Tokyo) 1999, 126, 905–911. [Google Scholar] [CrossRef]
- Koch, W.; Kaplan, D. A nomographic probit solution for the median effective dose (ED50). J. Immunol. 1950, 65, 7–16. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hervey, G.R. Determination of creatinine by the Jaffé reaction. Nature 1953, 171. [Google Scholar] [CrossRef]
- Kaltwasser, H.; Schlegel, H.G. NADH-dependent coupled enzyme assay for urease and other ammonia-producing systems. Anal. Biochem. 1966, 16, 132–138. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Okumura, S.; Koga, H.; Inouye, K.; Mizuki, E. Toxicity of Parasporin-4 and Health Effects of Pro-parasporin-4 Diet in Mice. Toxins 2014, 6, 2115-2126. https://doi.org/10.3390/toxins6072115
Okumura S, Koga H, Inouye K, Mizuki E. Toxicity of Parasporin-4 and Health Effects of Pro-parasporin-4 Diet in Mice. Toxins. 2014; 6(7):2115-2126. https://doi.org/10.3390/toxins6072115
Chicago/Turabian StyleOkumura, Shiro, Hironori Koga, Kuniyo Inouye, and Eiichi Mizuki. 2014. "Toxicity of Parasporin-4 and Health Effects of Pro-parasporin-4 Diet in Mice" Toxins 6, no. 7: 2115-2126. https://doi.org/10.3390/toxins6072115





