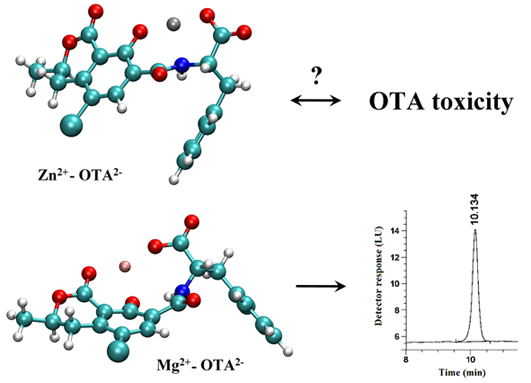
Further Aspects of Ochratoxin A-Cation Interactions: Complex Formation with Zinc Ions and a Novel Analytical Application of Ochratoxin A-Magnesium Interaction in the HPLC-FLD System
Abstract
:1. Introduction
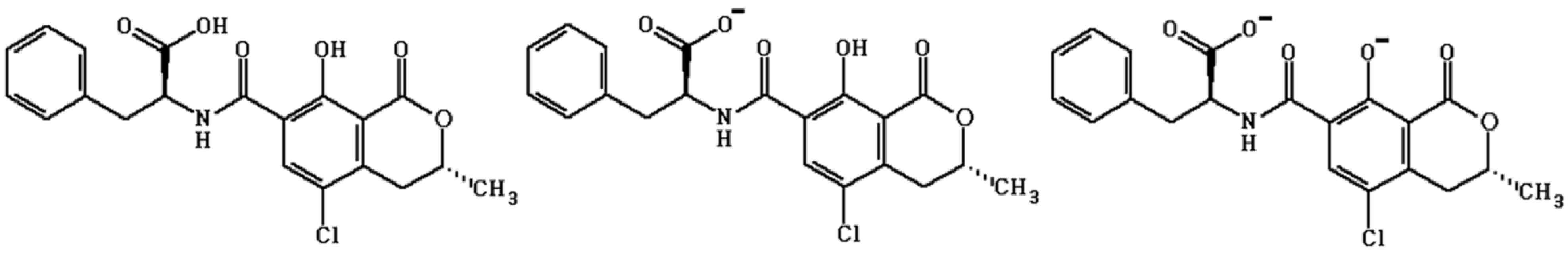
2. Results and Discussion
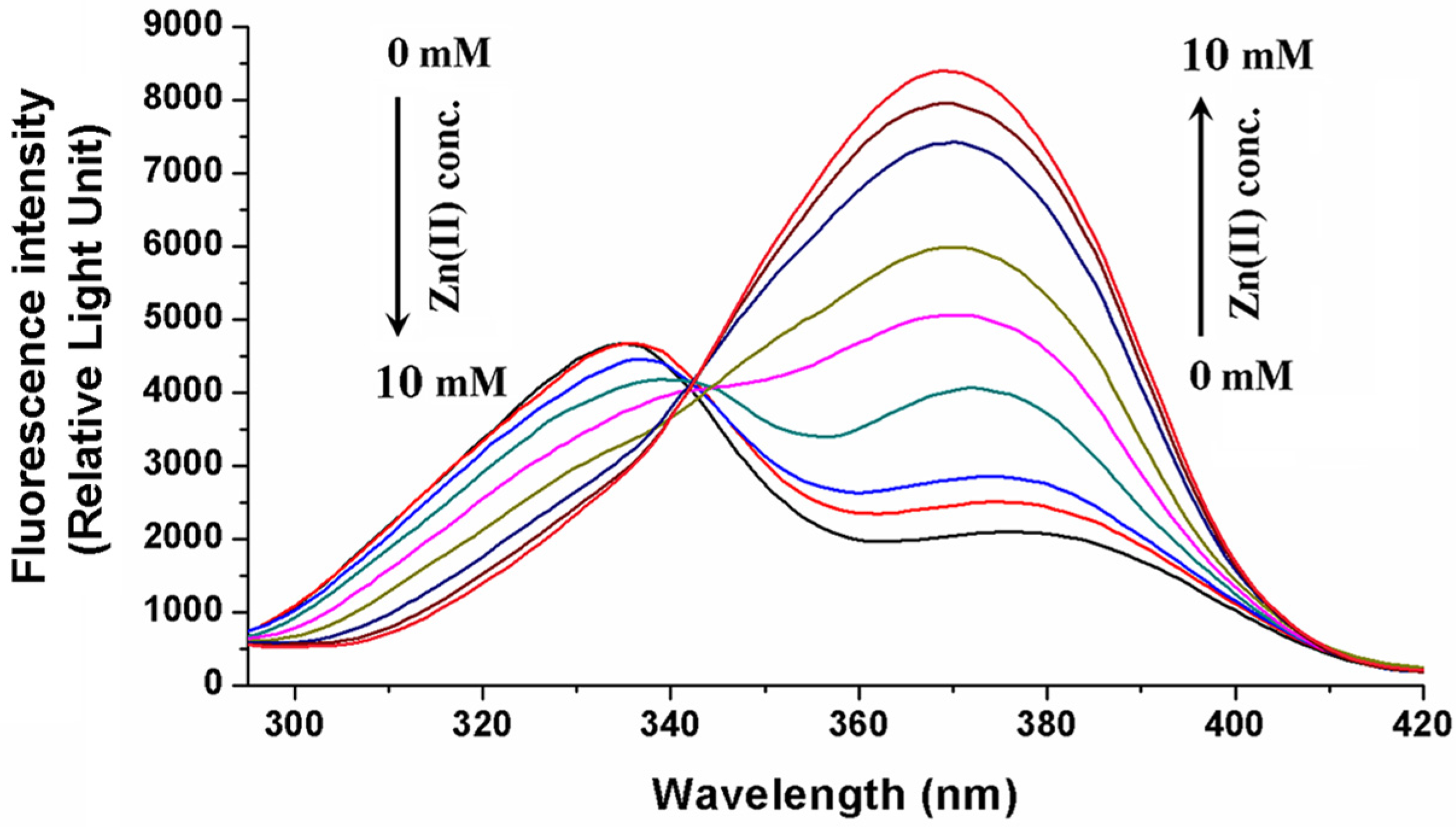
2.1. Interaction of OTA with Zinc and Magnesium Ions: Complex Stability and Thermodynamics

| Complex | Log K (25 °C) | ΔH (kJ·mol−1) | ΔS (J·mol−1·K−1) | ΔG (kJ∙mol−1; 25 °C) |
|---|---|---|---|---|
| Zn2+-OTA2− (TRIS-HCl) | 3.78 ± 0.01 | −10.4 ± 2.6 | 34.0 ± 9.8 | −21.6 ±0.1 |
| Mg2+-OTA2− (TRIS-HCl) | 3.40 ± 0.01 | −8.3 ± 0.4 | 37.6 ± 1.5 | −19.4 ± 0.1 |
| Mg2+-OTA2− (PBS) | 3.00 ± 0.01 | −3.0 ± 1.4 | 47.1 ± 4.5 | −17.1 ±0.1 |
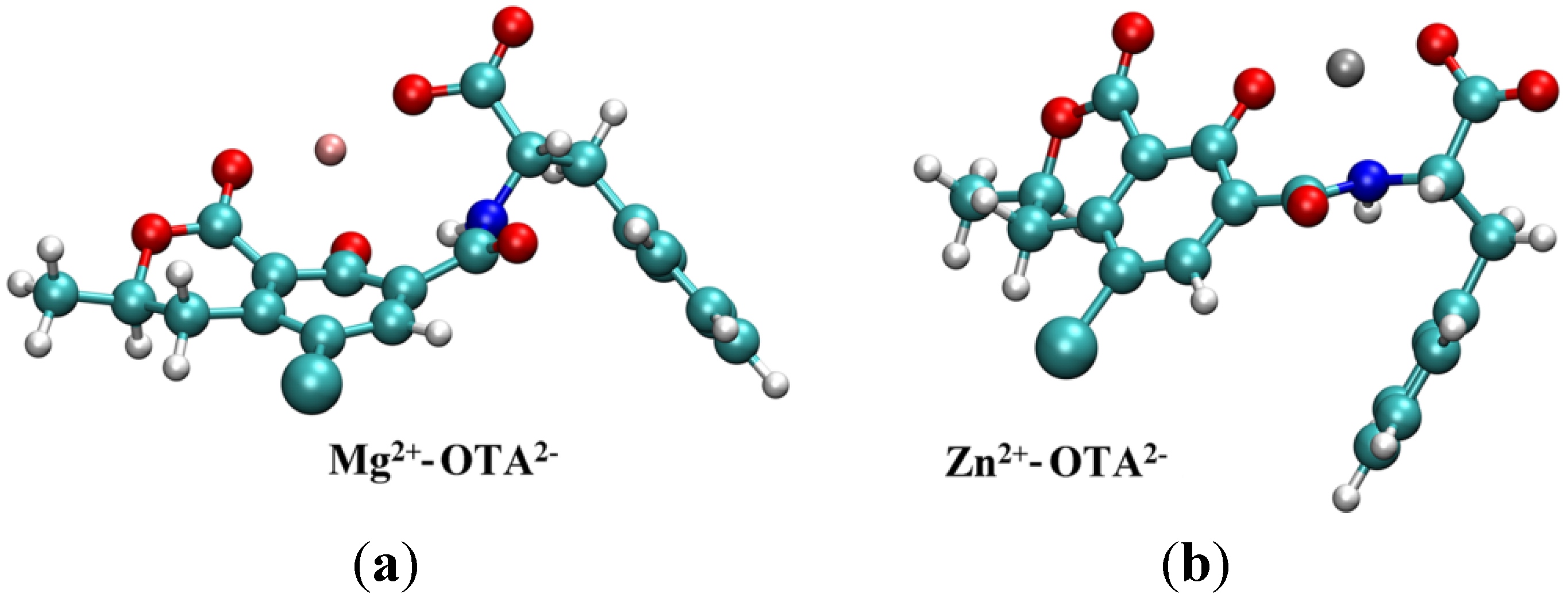
2.2. Molecular Modeling Studies
2.3. Influence of pH on the Mg2+-OTA2− Complex Formation

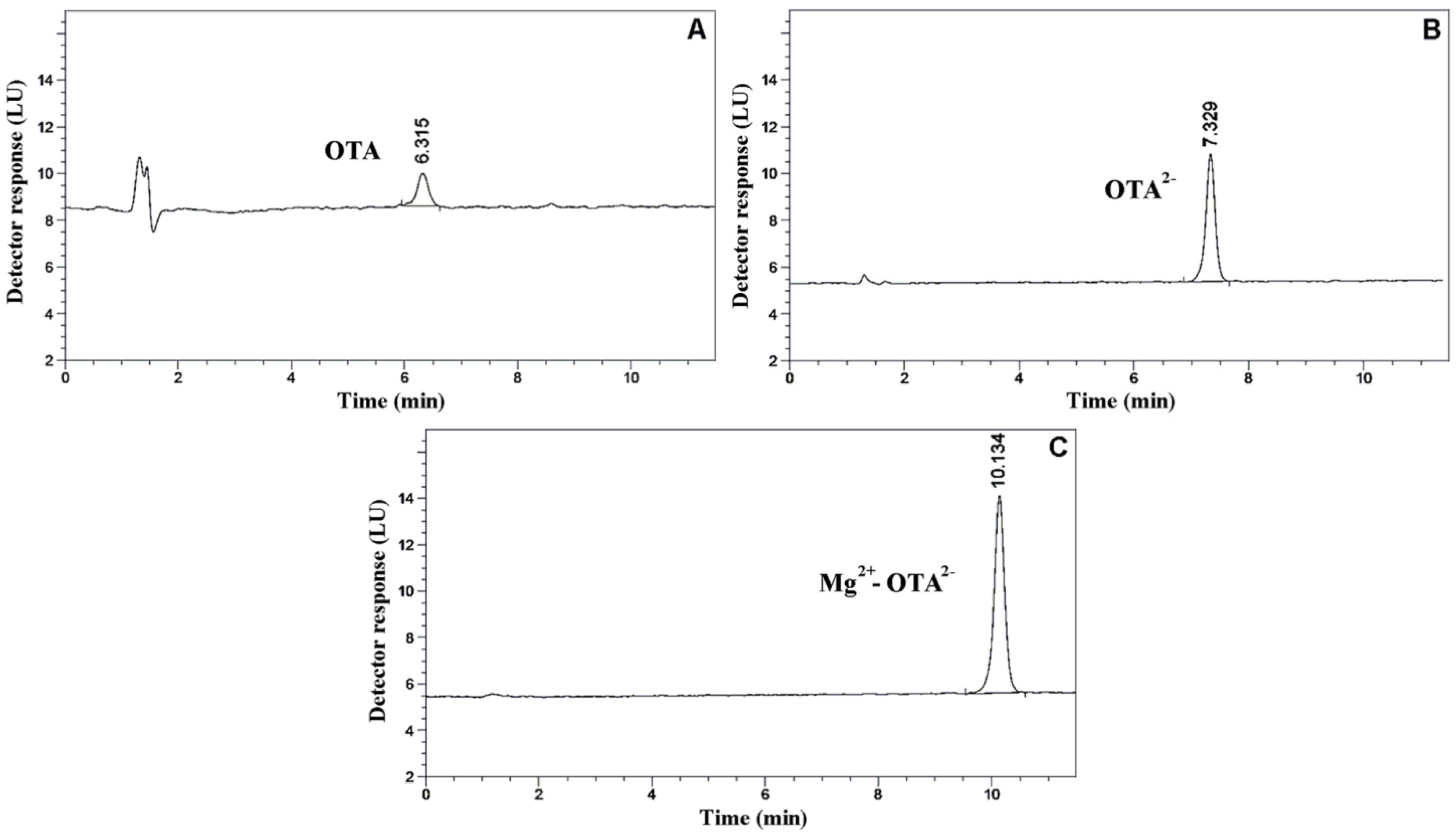
2.4. Analytical Application of the Mg2+-OTA2− Interaction on HPLC-FLD
3. Experimental Section
3.1. Reagents
3.2. Fluorescence Spectroscopic Investigations
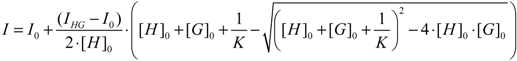
3.3. Molecular Modeling of Zn2+-OTA2− and Mg2+-OTA2− Complexes
3.4. Chromatographic Conditions on HPLC-FLD System
4. Conclusions
Acknowledgements
Authors Contributions
Conflicts of Interest
References
- Van Der Merwe, K.J.; Steyn, P.S.; Fourie, L. Part II. The constitution of ochratoxins A, B, and C, metabolites of Aspergillus ochraceus wilh. J. Chem. Soc. 1965, 7083–7088. [Google Scholar] [CrossRef]
- Pohland, A.E.; Nesheim, S.; Friedman, L. Ochratoxin A: A review. Pure Appl. Chem. 1992, 64, 1029–1046. [Google Scholar]
- Duarte, S.C.; Pena, A.; Lino, C.M. Ochratoxin A non-conventional exposure sources—A review. Microchem. J. 2009, 93, 115–120. [Google Scholar] [CrossRef]
- Amezqueta, S.; Gonzalez-Penas, E.; Murillo-Arbizu, M.; de Cerain, A.L. Ochratoxin A decontamination: A review. Food Contr. 2009, 20, 326–333. [Google Scholar] [CrossRef]
- Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef]
- Bozic, Z.; Duancic, V.; Kraus, O.; Skljarov, I. Balkan endemic nephropathy: Still a mysterious disease. Eur. J. Epidemiol. 1995, 11, 235–238. [Google Scholar] [CrossRef]
- Tatu, C.A.; Orem, W.H.; Finkelman, R.B.; Feder, G.L. The etiology of Balkan endemic nephropathy: Still more questions than answers. Environ. Health Perspect. 1998, 106, 689–700. [Google Scholar]
- Poór, M.; Kunsági-Máté, S.; Bencsik, T.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Flavonoid aglycones can compete with Ochratoxin A for human serum albumin: A new possible mode of action. Int. J. Biol. Macromol. 2012, 51, 279–283. [Google Scholar] [CrossRef]
- Hagelberg, S.; Hult, K.; Fuchs, R. Toxicokinetics of ochratoxin A in several species and its plasma-binding properties. J. Appl. Toxicol. 1989, 9, 91–96. [Google Scholar] [CrossRef]
- Studer-Rohr, I.; Schlatter, J.; Dietrich, D.R. Kinetic parameters and intraindividual fluctuations of Ochratoxin A plasma levels in human. Arch. Toxicol. 2000, 74, 499–510. [Google Scholar] [CrossRef]
- Rahimtula, A.D.; Béréziat, J.C.; Bussacchini-Griot, V.; Bartsch, H. Lipid peroxidation as a possible cause of ochratoxin A toxicity. Biochem. Pharmacol. 1988, 37, 4469–4477. [Google Scholar] [CrossRef]
- Omar, R.F.; Hasinoff, B.B.; Mejilla, F.; Rahimtula, A.D. Mechanism of ochratoxin A stimulated lipid peroxidation. Biochem. Pharmacol. 1990, 40, 1183–1191. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Matisz, G.; Li, Y.; Czibulya, Z.; Peles-Lemli, B.; Kőszegi, T. Interaction of alkali and alkaline earth ions with Ochratoxin A. J. Lumin. 2013, 135, 276–280. [Google Scholar] [CrossRef]
- Gibson, R.S. A historical review of progress in the assessment of dietary zinc intake as an indicator of population zinc status. Am. Soc. Nutr. Adv. Nutr. 2012, 3, 772–782. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer. Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef]
- Ranaldi, G.; Caprini, V.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc stores protect the intestinal epithelium from ochratoxin A toxicity. Toxicol. In Vitro 2009, 23, 1516–1521. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Xu, W.; Luo, Y.; Hao, J.; Shen, X.L.; Yang, X.; Li, X.; Huang, K. Zinc protects HepG2 cells against the oxidative damage and DNA damage induced by ochratoxin A. Toxicol. Appl. Pharmacol. 2013, 268, 123–131. [Google Scholar] [CrossRef]
- Scott, P.M. Methods of analysis for ochratoxin A. Adv. Exp. Med. Biol. 2002, 504, 117–134. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Meulenberg, E.P. Immunochemical methods for ochratoxin A detection: A review. Toxins 2012, 4, 244–266. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Czibulya, Z.; Li, Y.; Peles-Lemli, B.; Petrik, J.; Vladimir-Knezević, S.; Kőszegi, T. Fluorescence spectroscopic investigation of competitive interactions between ochratoxin A and 13 drug molecules for binding to human serum albumin. Luminescence 2013, 28, 726–733. [Google Scholar] [CrossRef]
- Valenta, H. Chromatographic methods for the determination of ochratoxin A in animal and human tissues and fluids. J. Chromatogr. A 1998, 815, 75–92. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S. Influence of different mobile phase compositions on detection of Ochratoxin A. Food Control 2013, 31, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Pittet, A.; Royer, D. Rapid, low cost thin-layer chromatographic screening method for the detection of ochratoxin A in green coffee at a control level of 10 microg/kg. J. Agric. Food Chem. 2002, 50, 243–247. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Galaverna, G.; Dossena, A.; Marchelli, R. Reversed-phase liquid chromatographic method for the determination of ochratoxin A in wine. J. Chromatogr. A 2004, 1024, 275–279. [Google Scholar]
- Toscani, T.; Moseriti, A.; Dossena, A.; Dall’Asta, C.; Simoncini, N.; Virgili, R. Determination of ochratoxin A in dry-cured meat products by a HPLC-FLD quantitative method. J. Chromatogr. B 2007, 855, 242–248. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 2006, 1763, 711–722. [Google Scholar] [CrossRef]
- Floriańczyk, B. Role of Zinc in nervous system cells. J. Pre Clinic. Clinic. Res. 2011, 5, 12–15. [Google Scholar]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef]
- Poór, M.; Li, Y.; Kunsági-Máté, S.; Varga, Z.; Hunyadi, A.; Dankó, B.; Chang, F.R.; Wu, Y.C.; Kőszegi, T. Protoapigenone derivatives: albumin binding properties and effects on HepG2 cells. J. Photochem. Photobiol. B 2013, 124, 20–26. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Hyperquad2006 version 3.1.60; Protonic Software: Leeds, UK, 2006. [Google Scholar]
- Cauët, E.; Bogatko, S.; Weare, J.H.; Fulton, J.L.; Schenter, G.K.; Bylaska, E.J. Structure and dynamics of the hydration shells of the Zn2+ ion from ab initio molecular dynamics and combined ab initio and classical molecular dynamics simulations. J. Chem. Phys. 2010, 132, 194502–194514. [Google Scholar] [CrossRef]
- Hawlicka, E.; Rybicki, M. MD Simulation of the Ion Solvation in Methanol-Water Mixtures. In Molecular Dynamics—Theoretical Developments and Applications in Nanotechnology and Energy; Wang, L., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ufimtsev, I.S.; Martinez, T.J. Quantum chemistry on graphical processing units. 3. analytical energy gradients and first principles molecular dynamics. J. Chem. Theory Comput. 2009, 5, 2619–2628. [Google Scholar] [CrossRef]
- Kästner, J.; Carr, J.M.; Keal, T.W.; Thiel, W.; Wander, A.; Sherwood, P. DL-FIND: An open-source geometry optimizer for atomistic simulations. J. Phys. Chem. A 2009, 113, 11856–11865. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154122. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comp. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef]
- Schafer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Poór, M.; Kuzma, M.; Matisz, G.; Li, Y.; Perjési, P.; Kunsági-Máté, S.; Kőszegi, T. Further Aspects of Ochratoxin A-Cation Interactions: Complex Formation with Zinc Ions and a Novel Analytical Application of Ochratoxin A-Magnesium Interaction in the HPLC-FLD System. Toxins 2014, 6, 1295-1307. https://doi.org/10.3390/toxins6041295
Poór M, Kuzma M, Matisz G, Li Y, Perjési P, Kunsági-Máté S, Kőszegi T. Further Aspects of Ochratoxin A-Cation Interactions: Complex Formation with Zinc Ions and a Novel Analytical Application of Ochratoxin A-Magnesium Interaction in the HPLC-FLD System. Toxins. 2014; 6(4):1295-1307. https://doi.org/10.3390/toxins6041295
Chicago/Turabian StylePoór, Miklós, Mónika Kuzma, Gergely Matisz, Yin Li, Pál Perjési, Sándor Kunsági-Máté, and Tamás Kőszegi. 2014. "Further Aspects of Ochratoxin A-Cation Interactions: Complex Formation with Zinc Ions and a Novel Analytical Application of Ochratoxin A-Magnesium Interaction in the HPLC-FLD System" Toxins 6, no. 4: 1295-1307. https://doi.org/10.3390/toxins6041295