Sensitive Quantification of Aflatoxin B1 in Animal Feeds, Corn Feed Grain, and Yellow Corn Meal Using Immunomagnetic Bead-Based Recovery and Real-Time Immunoquantitative-PCR
Abstract
:1. Introduction
2. Results and Discussion
2.1. Estimation of Aflatoxin Using CD-ELISA
2.2. Detection and Quantification of Aflatoxin B1 Using Real-Time Immune-Quantitative-PCR (RTiqPCR)
| Primers | Oligonucleotide sequences (5'-3') | Modification | PCR product size | Application of PCR product |
|---|---|---|---|---|
| pGL2A_Fwd | NH2-(C6)-GTTCGTCACATCTCATCTAC | 5'-amino | 560-bp | Tethered via SMCC to anti-AFB1 antibodies |
| pGL2A_Rev | TCGGGTGTAATCAGAATAGC | None | ||
| pGL2B_Fwd | GAACTGCCTGCGTCAGATTC | None | 101-bp | Real-time PCR fragment for quantification |
| pGL2B_Rev | AACCGTGATGGAATGGAACAAC | None |
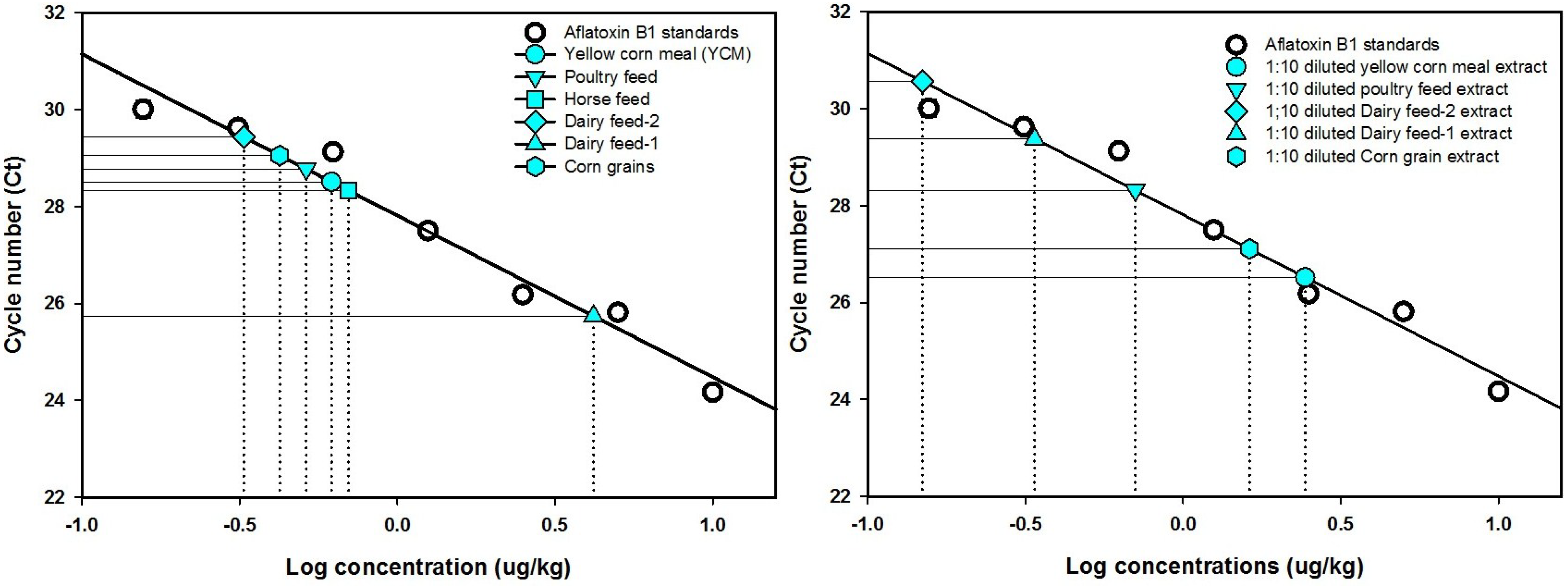

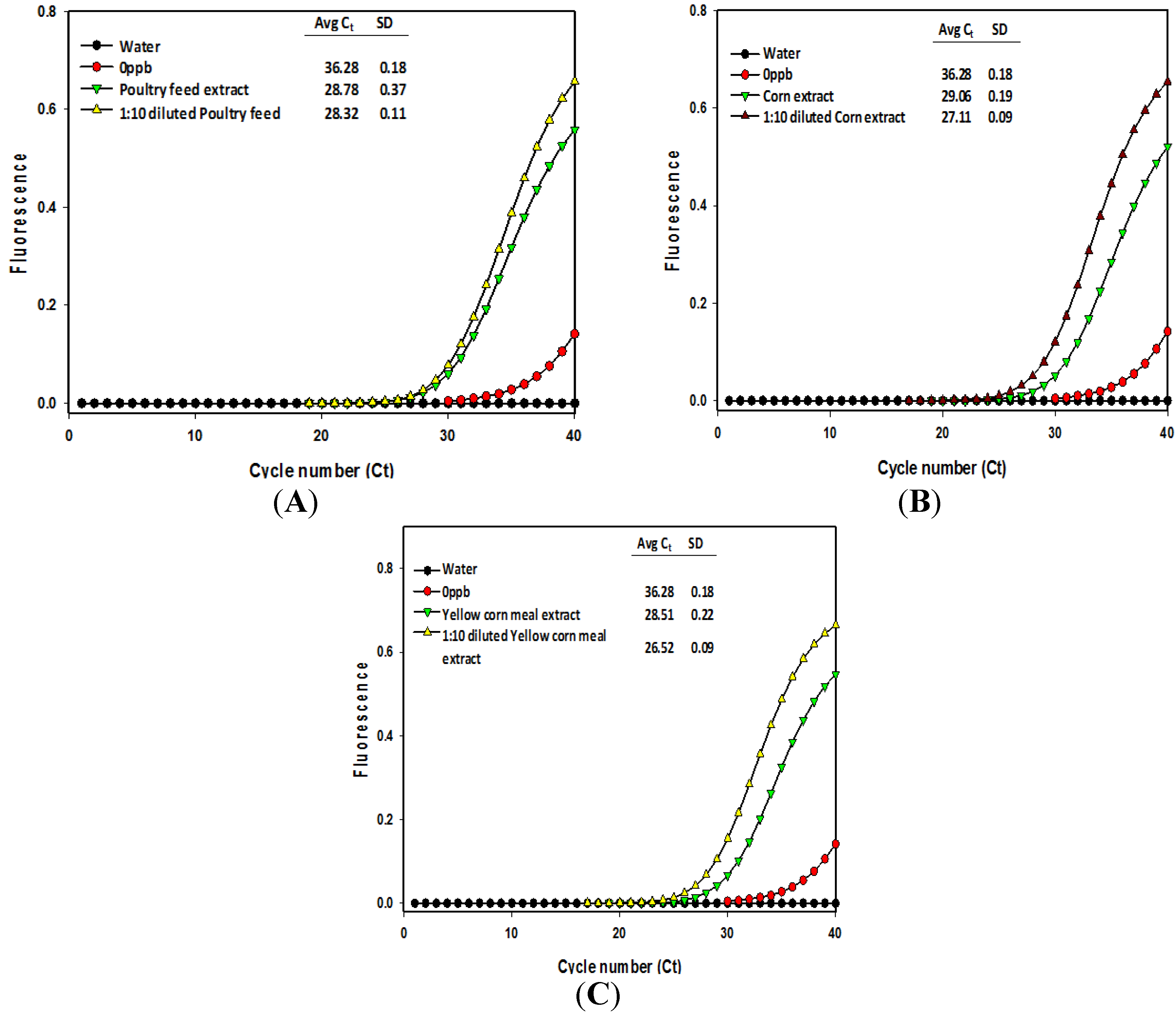
| Methanol/water (60:40) extracts | Cycle threshold (Ct) number 1 | Calculated aflatoxin (μg/kg) | Dilution-corrected aflatoxin (μg/kg) |
|---|---|---|---|
| Dairy feed 1 | 25.74 a | 4.18 | |
| Dairy feed 1 (1:10) | 29.38 b | 0.34 | 3.38 |
| Dairy feed 2 | 29.44 a | 0.32 | |
| Dairy feed 2 (1:10) | 30.57 b | 0.15 | 1.48 |
| Horse feed | 28.33 a | 0.70 | |
| Horse feed (1:10) | 32.46 b | 0.04 | 0.40 |
| Poultry feed | 28.78 a | 0.51 | |
| Poultry feed (1:10) | 28.32 a | 0.70 | 7.03 |
| Corn feed grains | 29.06 a | 0.42 | |
| Corn feed grains (1:10) | 27.11 b | 1.62 | 16.23 |
| Yellow corn meal | 28.51 a | 0.62 | |
| Yellow corn meal (1:10) | 26.52 b | 2.44 | 24.40 |
| Dairy feed 1 + AFB1 | 28.81 a | 0.50 | |
| Dairy feed 1 + AFB1 (1:10) | 27.95 b | 0.91 | 9.08 |
| Dairy feed 1 + AFB1 (1:40) | 30.1 c | 0.21 | 8.21 |
| Poultry feed + AFB1 | 29.61 a | 0.29 | |
| Poultry feed + AFB1 (1:10) | 26.97 b | 1.79 | 17.88 |
| Poultry feed + AFB1 (1:40) | 28.96 c | 0.45 | 18.06 |
| Horse feed + AFB1 | 29.63 a | 0.28 | |
| Horse feed + AFB1 (1:10) | 29.81 a | 0.25 | 2.51 |
| Horse feed + AFB1 (1:40) | 25.53 b | 4.84 | 193.53 |
| Yellow corn meal + AFB1 | 27.92 a | 0.93 | |
| Yellow corn meal + AFB1 (1:10) | 29.51 b | 0.31 | 3.09 |
| Yellow corn meal + AFB1 (1:40) | 29.41 b | 0.33 | 13.23 |

3. Experimental Section
3.1. Animal Feeds, Feed Grain, and Yellow Corn Meal Samples
3.2. Anti-Aflatoxin Antibodies and Magnetic Beads
3.3. Aflatoxin Extraction
3.4. Aflatoxin Detection Using CD-ELISA
3.5. Spiking of Feed and Food Samples
3.6. Preparation of Reporter DNA and Detection Antibodies
3.7. Sandwich Immunoassay and Immunomagnetic Bead Recovery of Aflatoxin from Sample Extracts
3.8. Dilution Protocols to Overcome Hook Effect
3.9. Detection and Quantification of Aflatoxin B1 Using Real-Time Immunoquantitative-PCR (RT-iqPCR)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAO/WHO. Animal feed impact on food safety report of the FAO/WHO expert meeting. Available online: http://www.who.int/foodsafety/publications/animal-feed/en/ (accessed on 28 November 2014).
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Mannon, J.; Johnson, E. Fungi down on the farm. New Sci. 1985, 105, 12–16. [Google Scholar]
- FAO. Worldwide regulations for mycotoxins in food and feed in 2003. Available online: http://www.fao.org/docrep/007/y5499e/y5499e00.HTM (accessed on 26 November 2014).
- Vardon, P.; McLaughlin, C.; Nardinelli, C. Mycotoxins: Risks in Plant, Animal, and Human Systems. Available online: http://www.trilogylab.com/uploads/Mycotoxin_CAST_Report.pdf (accessed on 28 November 2014).
- Dohlman, E. Mycotoxin hazards and regulations: Impacts on food and animal feed crop trade. Available online: http://www.ers.usda.gov/media/321551/aer828h_1_.pdf (accessed on 27 November 2014).
- Trenk, H.L.; Hartman, P.A. Effects of moisture content and temperature on aflatoxin production in corn. Appl. Microbiol. 1970, 19, 781–784. [Google Scholar] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.N.; Brijitha, N.; Raghavender, C.R. Aflatoxin contamination in insect damaged seeds of horsegram under storage. Mycotoxin Res. 2005, 21, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Shier, W.T.; Cartwright, R.D. Effect of temperature, rainfall and planting date on aflatoxin and fumonisin contamination in commercial Bt and non-Bt corn hybrids in Arkansas. Phytoprotection 2007, 88, 41–50. [Google Scholar] [CrossRef]
- Bircan, C.; Barringer, S.A.; Ulken, U.; Pehlivan, R. Increased aflatoxin contamination of dried figs in a drought year. Food Addit. Contam. Part A 2008, 25, 1400–1408. [Google Scholar] [CrossRef]
- Cotty, P.J. Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathology 1994, 125, 157–162. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Ehrlich, K.C.; Cleveland, T.E. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2003, 61, 83–93. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (IARC) Working Group: Lyon, France, 1993; Volume 56, pp. 245–395. [Google Scholar]
- Puschner, B. Mycotoxins. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 409–419. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses-An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Kemppainen, B.W.; Norred, W.P. Penetration of aflatoxins through isolated human epidermis. J. Toxicol. Environ. Health 1985, 15, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Vainio, H.; Heseltine, E.; Wilbourn, J. Priorities for future IARC monographs on the evaluation of carcinogenic risks to humans. Environ. Health Perspect. 1994, 102, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.B.; Kubena, L.F.; Elissalde, M.H.; Corrier, D.E.; Huff, W.E.; Rottinghaus, G.E.; Clement, B.A. Cocontamination of swine diets by aflatoxin and diacetoxyscirpenol. J. Vet. Diagn. Invest. 1991, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Miazzo, R.; Peralta, M.F.; Magnoli, C.; Salvano, M.; Ferrero, S.; Chiacchiera, S.M.; Carvalho, E.C.; Rosa, C.A.; Dalcero, A. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult. Sci. 2005, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rauber, R.H.; Dilkin, P.; Giacomini, L.Z.; Araujo de Almeida, C.A.; Mallmann, C.A. Performance of turkey poults fed different doses of aflatoxins in the diet. Poult. Sci. 2007, 86, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Diaz, G.J.; Sumers, J.D. Poultry Metabolic Disorders and Mycotoxins; University Books: Guelph, ON, Canada, 1995. [Google Scholar]
- Sano, T.; Smith, C.L.; Cantor, C.R. Immuno-PCR: Very sensitive antigen-detection by means of specific antibody-DNA conjugates. Science 1992, 258, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.M.; Adler, M.; Wacker, R. Immuno-PCR: High sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol. 2005, 23, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.; Muriana, P.M. Immunomagnetic bead-based recovery and real time quantitative PCR (RT iq-PCR) for sensitive quantification of aflatoxin B1. J. Microbiol. Methods 2011, 86, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rayner, E.T.; Dollear, F.G. Removal of aflatoxins from oilseed meals by extraction with aqueous isopropanol. J. Am. Oil Chem. Soc. 1968, 45, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Rayner, E.T.; Koltun, S.P.; Dollear, F.G. Solvent-extraction of aflatoxins from contaminated agricultural products. J. Am. Oil Chem. Soc. 1977, 54, A242. [Google Scholar] [CrossRef]
- Rodbard, D.; Feldman, Y.; Jaffe, M.L.; Miles, L.E. Kinetics of 2-site immunoradiometric (sandwich) assays-II. Studies on nature of high-dose hook effect. Immunochem. 1978, 15, 77–82. [Google Scholar] [CrossRef]
- Fernando, S.A.; Wilson, G.S. Studies of the hook effect in the one-step sandwich immunoassay. J. Immunol. Methods 1992, 151, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Killeen, A.A.; Ramey, M.L.; Dean, J.J. High-dose hook effect in an immunoluminometric thyrotropin assay: The open-faced sandwich artifact. Ann. Clin. Biochem. 1993, 30, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Saryan, J.A.; Garrett, P.E.; Kurtz, S.R. Failure to detect extremely high-levels of serum IgE with an immunoradiometric assay. Annal. Allergy 1989, 63, 322–324. [Google Scholar]
- Butch, A.W. Dilution protocols for detection of hook effects/prozone phenomenon. Clin. Chem. 2000, 46, 1719–1721. [Google Scholar] [PubMed]
- Tang, M.E.; Standage, J.C. Dilution protocols for detection of hook effects/prozone phenomenon (Letter to the editor). Clin. Chem. 2000, 46, 1722–1722. [Google Scholar]
- Lee, N.A.; Wang, S.; Allan, R.D.; Kennedy, I.R. A rapid aflatoxin B1 ELISA: Development and validation with reduced matrix effects for peanuts, corn, pistachio, and soybeans. J. Agric. Food Chem. 2004, 52, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Huang, Y.L.; Lai, S.C.; Huang, Y.Y.; Shalo, M.F. Detection of Clostridium botulinum neurotoxin type-a using immuno-PCR. Lett. Appl. Microbiol. 2001, 32, 321–352. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babu, D.; Muriana, P.M. Sensitive Quantification of Aflatoxin B1 in Animal Feeds, Corn Feed Grain, and Yellow Corn Meal Using Immunomagnetic Bead-Based Recovery and Real-Time Immunoquantitative-PCR. Toxins 2014, 6, 3223-3237. https://doi.org/10.3390/toxins6123223
Babu D, Muriana PM. Sensitive Quantification of Aflatoxin B1 in Animal Feeds, Corn Feed Grain, and Yellow Corn Meal Using Immunomagnetic Bead-Based Recovery and Real-Time Immunoquantitative-PCR. Toxins. 2014; 6(12):3223-3237. https://doi.org/10.3390/toxins6123223
Chicago/Turabian StyleBabu, Dinesh, and Peter M. Muriana. 2014. "Sensitive Quantification of Aflatoxin B1 in Animal Feeds, Corn Feed Grain, and Yellow Corn Meal Using Immunomagnetic Bead-Based Recovery and Real-Time Immunoquantitative-PCR" Toxins 6, no. 12: 3223-3237. https://doi.org/10.3390/toxins6123223





