Uptake and Processing of the Cytolethal Distending Toxin by Mammalian Cells
Abstract
:1. Introduction
2. Synthesis and Secretion of the Cdt
2.1. Sec-Dependent Secretion
2.2. Other Cdt Delivery Mechanisms
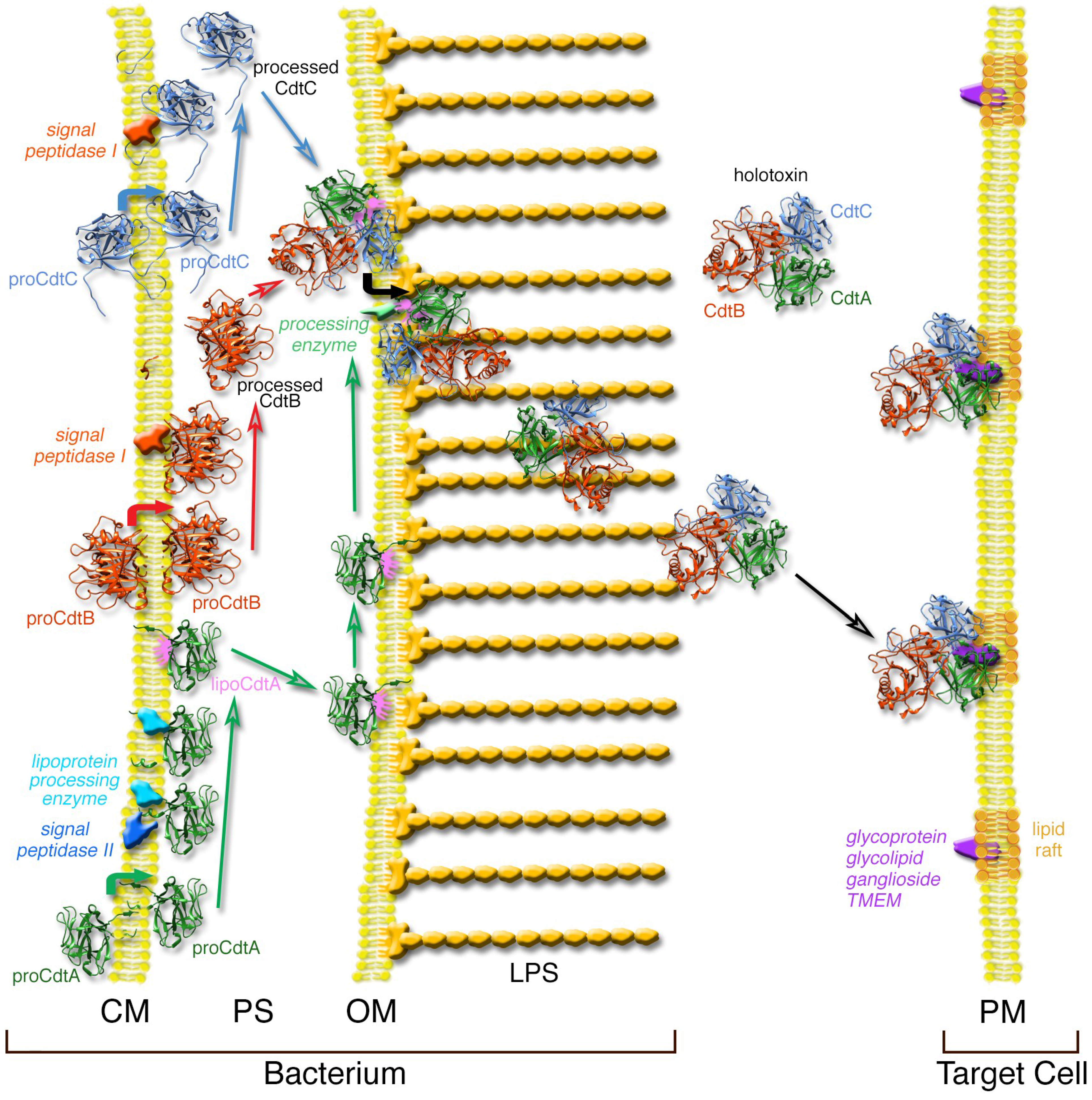
3. Steps in the Intoxication Process
3.1. Step 1—Recognition of Target Cells
3.2. Step 2—Endocytosis
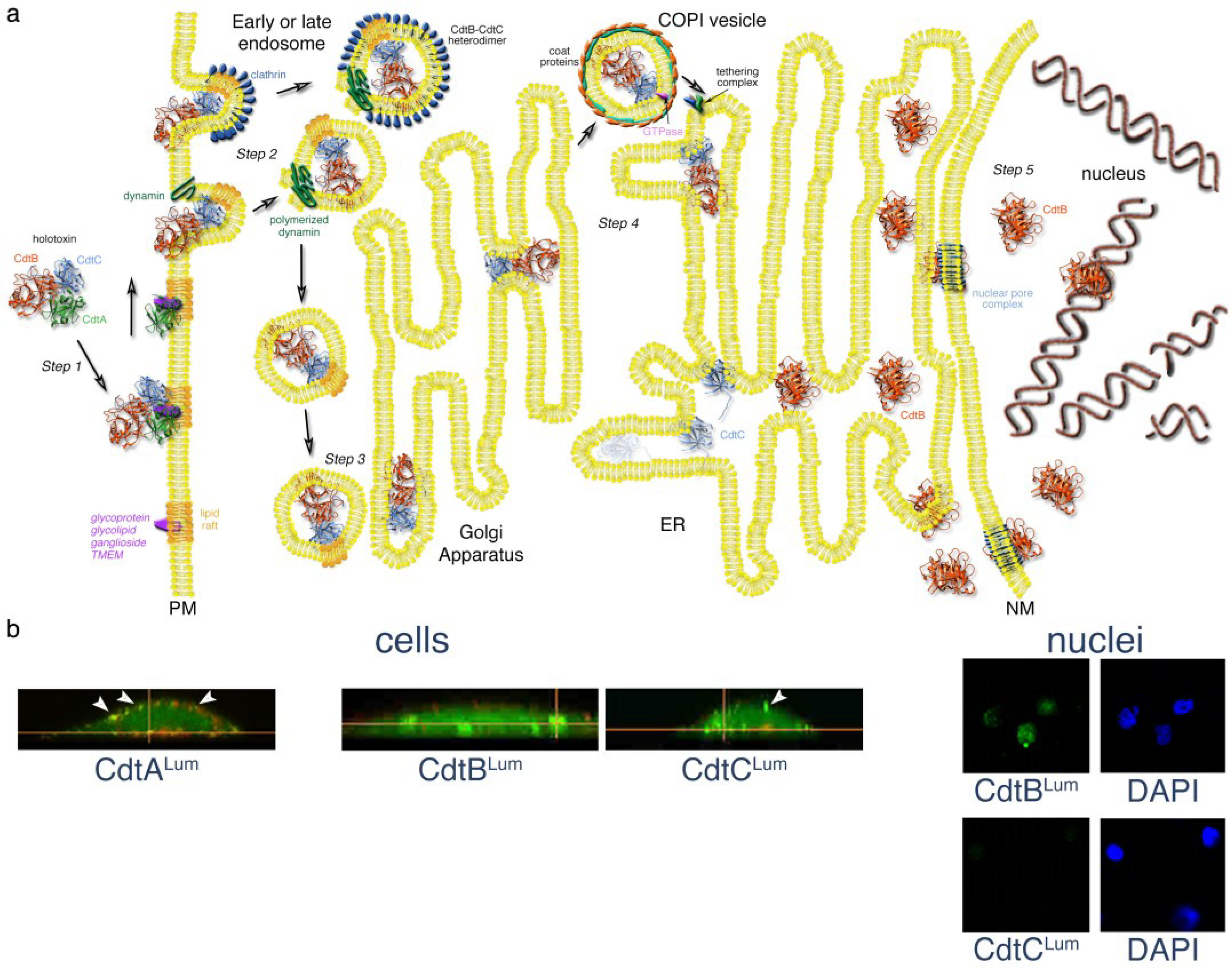
3.3. Step 3—Trafficking to the Golgi Apparatus
3.4. Step 4—Passage to the Endoplasmic Reticulum
3.5. Step 5—Entering the Nucleus
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- DiRienzo, J.M. Cytolethal distending toxin: A unique variation on the AB toxin paradigm. New J. Sci. 2014, in press. [Google Scholar]
- Elwell, C.A.; Dreyfus, L.A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 2000, 37, 952–963. [Google Scholar] [PubMed]
- Lara-Tejero, M.; Galán, J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef]
- Dlakic, M. Is CdtB a nuclease or a phosphatase? Science 2001, 291, 547. [Google Scholar] [CrossRef]
- Hofmann, K.; Tomiuk, S.; Wolff, G.; Stoffel, W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc. Natl. Acad. Sci. USA 2000, 97, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Damek-Poprawa, M.; Jang, J.Y.; Volgina, A.; Korostoff, J.; DiRienzo, J.M. Localization of Aggregatibacter actinomycetemcomitans cytolethal distending toxin subunits during intoxication of live cells. Infect. Immun. 2012, 80, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Mezal, E.H.; Bae, D.; Khan, A.A. Detection and functionality of the CdtB, PltA and PltB from Salmonella enterica serovar Javiana. Pathog. Dis. 2014. [Google Scholar] [CrossRef]
- Spano, S.; Ugalde, J.E.; Galán, J.E. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe. 2008, 3, 30–38. [Google Scholar] [CrossRef]
- Song, J.; Gao, X.; Galán, J.E. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 2013, 499, 350–354. [Google Scholar] [CrossRef]
- Ueno, Y.; Ohara, M.; Kawamoto, T.; Fujiwara, T.; Komatsuzawa, H.; Oswald, E.; Sugai, M. Biogenesis of the Actinobacillus actinomycetemcomitans cytolethal distending toxin holotoxin. Infect. Immun. 2006, 74, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Pugsley, A.P. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 1993, 57, 50–108. [Google Scholar] [PubMed]
- Hayashi, S.; Wu, H.C. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 1990, 22, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, J. The Sec-dependent pathway. Res. Microbiol. 2013, 164, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Frisk, A.; Lebens, M.; Johansson, C.; Ahmed, H.; Svensson, L.; Ahlman, K.; Lagergard, T. The role of different protein components from the Haemophilus ducreyi cytolethal distending toxin in the generation of cell toxicity. Microb. Pathog. 2001, 30, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Besack, D.; McKay, T.; Pankoski, L.; Zekavat, A.; Demuth, D.R. Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt): Evidence that the holotoxin is composed of three subunits: CdtA, CdtB, and CdtC. J. Immunol. 2004, 172, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, B.; Rompikuntal, P.K.; Vaitkevicius, K.; Song, T.; Mizunoe, Y.; Uhlin, B.E.; Guerry, P.; Wai, S.N. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Rompikuntal, P.K.; Thay, B.; Khan, M.K.; Alanko, J.; Penttinen, A.M.; Asikainen, S.; Wai, S.N.; Oscarsson, J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin (CDT) from Aggregatibacter actinomycetemcomitans. Infect Immun. 2011, 80, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Haghjoo, E.; Galán, J.E. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 4614–4619. [Google Scholar]
- Gorvel, J.P.; Meresse, S. Maturation steps of the Salmonella-containing vacuole. Microbes Infect. 2001, 3, 1299–1303. [Google Scholar] [CrossRef]
- Holden, D.W. Trafficking of the Salmonella vacuole in macrophages. Traffic 2002, 3, 161–169. [Google Scholar] [PubMed]
- McSweeney, L.A.; Dreyfus, L.A. Carbohydrate-binding specificity of the Escherichia coli cytolethal distending toxin CdtA-II and CdtC-II subunits. Infect. Immun. 2005, 73, 2051–2060. [Google Scholar] [CrossRef]
- Mise, K.; Akifusa, S.; Watarai, S.; Ansai, T.; Nishihara, T.; Takehara, T. Involvement of ganglioside GM3 in G2/M cell cycle arrest of human monocytic cells induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect. Immun. 2005, 73, 4846–4852. [Google Scholar] [CrossRef]
- Gargi, A.; Tamilselvam, B.; Powers, B.; Prouty, M.G.; Lincecum, T.; Eshraghi, A.; Maldonado-Arocho, F.J.; Wilson, B.A.; Bradley, K.A.; Blanke, S.R. Cellular interactions of the cytolethal distending toxins from Escherichia coli and Haemophilus ducreyi. J. Biol. Chem. 2013, 288, 7492–7505. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Volgina, A.; Huang, C.M.; Korostoff, J.; DiRienzo, J.M. Characterization of point mutations in the cdtA gene of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 2005, 58, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.; Maldonado-Arocho, F.J.; Gargi, A.; Cardwell, M.M.; Prouty, M.G.; Blanke, S.R.; Bradley, K.A. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. J. Biol. Chem. 2010, 285, 18199–18207. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.E.; Guimaraes, C.P.; Varadarajan, M.; Park, A.S.; Wuethrich, I.; Godarova, A.; Kotecki, M.; Cochran, B.H.; Spooner, E.; Ploegh, H.L.; et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science 2009, 326, 1231–1235. [Google Scholar] [CrossRef]
- Carette, J.E.; Guimaraes, C.P.; Wuethrich, I.; Blomen, V.A.; Varadarajan, M.; Sun, C.; Bell, G.; Yuan, B.; Muellner, M.K.; Nijman, S.M.; et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat. Biotechnol. 2011, 29, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Nešić, D.; Hsu, Y.; Stebbins, C.E. Assembly and function of a bacterial genotoxin. Nature 2004, 429, 429–433. [Google Scholar] [CrossRef]
- Yamada, T.; Komoto, J.; Saiki, K.; Konishi, K.; Takusagawa, F. Variation of loop sequence alters stability of cytolethal distending toxin (CDT): Crystal structure of CDT from Actinobacillus actinomycetemcomitans. Protein Sci. 2006, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Bandelac, G.; Volgina, A.; Korostoff, J.; DiRienzo, J.M. Role of aromatic amino acids in receptor binding activity and subunit assembly of the cytolethal distending toxin of Aggregatibacter actinomycetemcomitans. Infect. Immun. 2008, 76, 2812–2821. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Nešić, D.; Stebbins, C.E. Comparative structure-function analysis of cytolethal distending toxins. Proteins 2006, 62, 421–434. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Besack, D.; McKay, T.; Zekavat, A.; Otis, L.; Jordan-Sciutto, K.; Shenker, B.J. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell. Microbiol. 2006, 8, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Brown, A.; Walker, L.; Besack, D.; Zekavat, A.; Wrenn, S.; Krummenacher, C.; Shenker, B.J. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J. Biol. Chem. 2009, 284, 10650–10658. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Teter, K.; Lilley, B.N.; Stenerlow, B.; Holmes, R.K.; Ploegh, H.L.; Sandvig, K.; Thelestam, M.; Frisan, T. Cellular internalization of cytolethal distending toxin: A new end to a known pathway. Cell. Microbiol. 2005, 7, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Mahammad, S.; Parmryd, I. Cholesterol homeostasis in T cells. Methyl-β-cyclodextrin treatment results in equal loss of cholesterol from Triton X-100 soluble and insoluble fractions. Biochim. Biophys. Acta 2008, 1778, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.D.; Lai, C.K.; Lin, Y.H.; Hsieh, J.T.; Sing, Y.T.; Chang, Y.C.; Chen, K.C.; Wang, W.C.; Su, H.L.; Lai, C.H. Cholesterol depletion reduces entry of Campylobacter jejuni cytolethal distending toxin and attenuates intoxication of host cells. Infect. Immun. 2011, 79, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [PubMed]
- Sandvig, K.; van Deurs, B. Delivery into cells: Lessons learned from plant and bacterial toxins. Gene Ther. 2005, 12, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Liu, S.; Cosson, P.; Leppla, S.H.; van der Goot, F.G. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 2003, 160, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Rojas, R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell. Biol. 2006, 7, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Skotland, T.; van Deurs, B.; Klokk, T.I. Retrograde transport of protein toxins through the Golgi apparatus. Histochem. Cell Biol. 2013, 140, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Bratti, X.; Chaves-Olarte, E.; Lagergard, T.; Thelestam, M. Cellular internalization of cytolethal distending toxin from Haemophilus ducreyi. Infect. Immun. 2000, 68, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The biology of the cytolethal distending toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Sever, S. Dynamin and endocytosis. Curr. Opin. Cell Biol. 2002, 14, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, J.E.; Schmid, S.L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature 1995, 374, 190–192. [Google Scholar] [CrossRef] [PubMed]
- van der Bliek, A.M.; Redelmeier, T.E.; Damke, H.; Tisdale, E.J.; Meyerowitz, E.M.; Schmid, S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993, 122, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Brandizzi, F.; Barlowe, C. Organization of the ER-Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell. Biol. 2013, 14, 382–392. [Google Scholar] [PubMed]
- De Matteis, M.A.; Luini, A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 2008, 9, 273–284. [Google Scholar] [CrossRef]
- Papanikou, E.; Glick, B.S. Golgi compartmentation and identity. Curr. Opin. Cell Biol. 2014, 29C, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Spang, A. Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Gillespie, E.J.; Ho, C.L.; Balaji, K.; Clemens, D.L.; Deng, G.; Wang, Y.E.; Elsaesser, H.J.; Tamilselvam, B.; Gargi, A.; Dixon, S.D.; et al. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proc. Natl. Acad. Sci. USA 2013, 110, E4904–E4912. [Google Scholar] [CrossRef] [PubMed]
- Lencer, W.I.; Strohmeier, G.; Moe, S.; Carlson, S.L.; Constable, C.T.; Madara, J.L. Signal transduction by cholera toxin: Processing in vesicular compartments does not require acidification. Am. J. Physiol. 1995, 269, G548–G557. [Google Scholar] [PubMed]
- Mallard, F.; Antony, C.; Tenza, D.; Salamero, J.; Goud, B.; Johannes, L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 1998, 143, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.C.; Dascher, C.; Roberts, L.M.; Lord, J.M.; Balch, W.E. Ricin cytotoxicity is sensitive to recycling between the endoplasmic reticulum and the Golgi complex. J. Biol. Chem. 1995, 270, 20078–20083. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; DiRienzo, J.M. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell. Microbiol. 2002, 4, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Popoff, V. Tracing the retrograde route in protein trafficking. Cell 2008, 135, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Burd, C.G. Physiology and pathology of endosome-to-Golgi retrograde sorting. Traffic 2011, 12, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Attar, N.; Cullen, P.J. The retromer complex. Adv. Enzyme Regul. 2010, 50, 216–236. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Hurley, J.H. Retromer. Curr. Opin. Cell Biol. 2008, 20, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Popoff, V.; Mardones, G.A.; Tenza, D.; Rojas, R.; Lamaze, C.; Bonifacino, J.S.; Raposo, G.; Johannes, L. The retromer complex and clathrin define an early endosomal retrograde exit site. J. Cell Sci. 2007, 120, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Huttner, W.B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J. Cell Biol. 1987, 105, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, J.N.; Schroeder, L.K.; Fotouhi, M.; Dokainish, H.; Ioannou, M.S.; Girard, M.; Summerfeldt, N.; Melancon, P.; McPherson, P.S. Scyl1 scaffolds class II Arfs to specific subcomplexes of coatomer through the γ-COP appendage domain. J. Cell Sci. 2014, 127, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Xu, W.; Stamnes, M. In vitro reconstitution of ARF-regulated cytoskeletal dynamics on Golgi membranes. Met. Enzymol. 2005, 404, 345–358. [Google Scholar]
- Bremser, M.; Nickel, W.; Schweikert, M.; Ravazzola, M.; Amherdt, M.; Hughes, C.A.; Sollner, T.H.; Rothman, J.E.; Wieland, F.T. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 1999, 96, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Echard, A.; Jollivet, F.; Martinez, O.; Lacapere, J.J.; Rousselet, A.; Janoueix-Lerosey, I.; Goud, B. Interaction of a Golgi-associated kinesin-like protein with Rab. Science 1998, 279, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Walch-Solimena, C.; Collins, R.N.; Novick, P.J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997, 137, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Girod, A.; Storrie, B.; Simpson, J.C.; Johannes, L.; Goud, B.; Roberts, L.M.; Lord, J.M.; Nilsson, T.; Pepperkok, R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1999, 1, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; AbuJarour, R.J.; Draper, R.K. Evidence that the transport of ricin to the cytoplasm is independent of both Rab6A and COPI. J. Cell Sci. 2003, 116, 3503–3510. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.H.; Moe, D.; Jamnadas, M.; Tatulian, S.A.; Teter, K. The pertussis toxin S1 subunit is a thermally unstable protein susceptible to degradation by the 20S proteasome. Biochemistry 2006, 45, 13734–13740. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.H.; Scaglione, P.; Taylor, M.; Nemec, K.N.; Tuthill, S.; Moe, D.; Holmes, R.K.; Tatulian, S.A.; Teter, K. Conformational instability of the cholera toxin A1 polypeptide. J. Mol. Biol. 2007, 374, 1114–1128. [Google Scholar] [PubMed]
- Teter, K.; Holmes, R.K. Inhibition of endoplasmic reticulum-associated degradation in CHO cells resistant to cholera toxin, Pseudomonas aeruginosa exotoxin A, and ricin. Infect. Immun. 2002, 70, 6172–6179. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, F.G.; Guimaraes, C.P.; Maruyama, T.; Carette, J.E.; Lory, S.; Brummelkamp, T.R.; Ploegh, H.L. GPR107, a G-protein-coupled receptor essential for intoxication by Pseudomonas aeruginosa exotoxin A, localizes to the Golgi and is cleaved by furin. J. Biol. Chem. 2014, 289, 24005–24018. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, P.; Nemec, K.N.; Burlingame, K.E.; Grabon, A.; Huerta, J.; Navarro-Garcia, F.; Tatulian, S.A.; Teter, K. Structural characteristics of the plasmid-encoded toxin from enteroaggregative Escherichia coli. Biochemistry 2008, 47, 9582–9591. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.; Dixon, S.D.; Tamilselvam, B.; Kim, E.J.; Gargi, A.; Kulik, J.C.; Damoiseaux, R.; Blanke, S.R.; Bradley, K.A. Cytolethal distending toxins require components of the ER-associated degradation pathway for host cell entry. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef]
- Guerra, L.; Nemec, K.N.; Massey, S.; Tatulian, S.A.; Thelestam, M.; Frisan, T.; Teter, K. A novel mode of translocation for cytolethal distending toxin. Biochim. Biophys. Acta 2008, 1793, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Chinnapen, D.J.; Aamar, E.; te Welscher, Y.M.; Lencer, W.I.; Massol, R. Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins. Front. Cell. Infect. Microbiol. 2012, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wernick, N.L.B.; Chinnapen, D.J.F.; Cho, J.A.; Lencer, W.I. Cholera toxin: An intracellular journey into the cytosol by way of endoplasmic reticulum. Toxins 2010, 2, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Lara-Tejero, M.; Galán, J.E. Cytolethal distending toxin: Limited damage as a strategy to modulate cellular functions. Trends Microbiol. 2002, 10, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y. How proteins are transported from cytoplasm to the nucleus. J. Biochem. 1997, 121, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, J.; Heitman, J.; Hall, M.N. Nuclear protein localization. Biochim. Biophys. Acta 1991, 1071, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Dingwall, C.; Laskey, R.A. Nuclear targeting sequences—A consensus? Trends Biochem. Sci. 1991, 16, 478–481. [Google Scholar]
- Christophe, D.; Christophe-Hobertus, C.; Pichon, B. Nuclear targeting of proteins: How many different signals? Cell Signal. 2000, 12, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, S.; Ohara, M.; Ueno, Y.; Ikura, M.; Kurihara, H.; Komatsuzawa, H.; Oswald, E.; Sugai, M. An N-terminal segment of the active component of the bacterial genotoxin cytolethal distending toxin B (CDTB) directs CDTB into the nucleus. J. Biol. Chem. 2003, 278, 50671–50681. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, L.A.; Dreyfus, L.A. Nuclear localization of the Escherichia coli cytolethal distending toxin CdtB subunit. Cell. Microbiol. 2004, 6, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Torgersen, M.L.; Engedal, N.; Skotland, T.; Iversen, T.G. Protein toxins from plants and bacteria: Probes for intracellular transport and tools in medicine. FEBS Lett. 2010, 584, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiRienzo, J.M. Uptake and Processing of the Cytolethal Distending Toxin by Mammalian Cells. Toxins 2014, 6, 3098-3116. https://doi.org/10.3390/toxins6113098
DiRienzo JM. Uptake and Processing of the Cytolethal Distending Toxin by Mammalian Cells. Toxins. 2014; 6(11):3098-3116. https://doi.org/10.3390/toxins6113098
Chicago/Turabian StyleDiRienzo, Joseph M. 2014. "Uptake and Processing of the Cytolethal Distending Toxin by Mammalian Cells" Toxins 6, no. 11: 3098-3116. https://doi.org/10.3390/toxins6113098



