The Toxicological Impacts of the Fusarium Mycotoxin, Deoxynivalenol, in Poultry Flocks with Special Reference to Immunotoxicity
Abstract
:1. Introduction
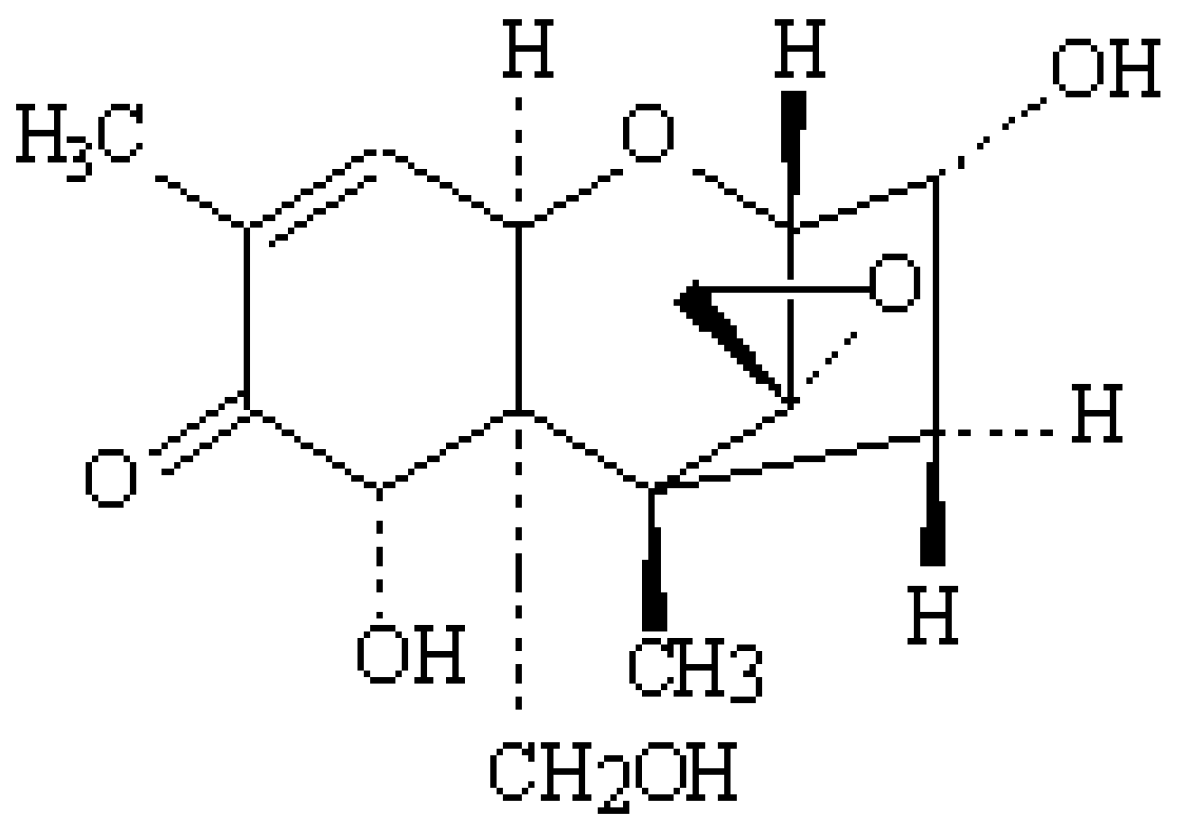
2. Occurrence of DON in Poultry Feed
3. Effects on Growth Performance
| Deoxynivalenol (mg/kg diet) Birds (species and sex) | Duration of exposure (days) | Performance traits | Reference |
|---|---|---|---|
| Up to 15 mg/kg diet Broiler (male or mixed sex) | 21–42 | No effects on performance | [16,17,18,35,36,37,38,39,40,41] |
| 10–15 mg/kg diet Broiler (male or mixed sex) | 21–42 | Reduced feed intake, weight gain and feed efficiency | [13,29,30,31] |
| 20 mg/kg diet Turkey poults (female) | 21 | No effects on performance | [45] |
| 2–3 mg/kg Laying hens (aged 48 weeks) | 56 | No effects on performance and egg production | [42] |
| 0.02 mg/kg Lohman Brown laying hens | 42 | Reduced feed intake | [14] |
4. Effects on the Immune System and Internal Organs
5. Effects on Gut Health
6. Effects on the Immune Responses
6.1. Humoral Immunity
| Deoxynivalenol (mg/kg diet) Birds (species and sex) | Duration of exposure (days) | Observed effects | Reference |
|---|---|---|---|
| Up to 12.21 mg/kg diet Broiler (male) | 35 | NDV increased at week 2 and 4 NDV decreased at week 5 IBV decreased at week 5 IBV not affected at week 2, 4, 5 | [60] |
| 10 mg/kg diet Broiler (male) | 35 | IBV decreased at week 5 | [13] |
| 3.5–14 mg/kg diet Broiler (male) | 35 | NDV decreased | [30] |
| 4.7–8.3 mg/kg diet Broiler (male) | 14 | IBV not affected | [38] |
| 12.6 mg/kg diet Broiler breeder | 84 | IBV decreased NDV not affected | [61] |
| 18 mg/kg White leghorn chicks | 72 | NDV not affected | [59] |
| 12.3 mg/kg Laying hens | 112 | NDV decreased | [14] |
6.2. Cellular Immunity
7. Effects on Pro-inflammatory Cytokines
8. Conclusions
References
- Dersjant-Li, Y.; Verstegen, M.W.A.; Gerrits, W.J.J. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 2003, 16, 223–239. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Pettersson, H.; Lindberg, J.E. Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch. Tierernähr. 2003, 57, 335–345. [Google Scholar]
- Desjardins, A.E. Mechanism of Action of Trichothecenes. In Fusarium Mycotoxins: Chemistry, Genetics and Biology; APS Press: St. Paul, MN, USA, 2006; pp. 53–54. [Google Scholar]
- Oswald, I.P.; Marin, D.E.; Bouhet, S.; Pinton, P.; Taranu, I.; Accensi, F. Immunotoxicological risk of mycotoxins for domestic animals. Food Add. Contam. 2005, 22, 354–360. [Google Scholar] [CrossRef]
- Swamy, H.V.L.N.; Smith, T.K.; Karrow, N.A.; Boermans, H.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on growth and immunological parameters of broiler chickens. Poult. Sci. 2004, 83, 533–543. [Google Scholar]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B 2000, 3, 109–143. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef]
- Becker, C.; Reiter, M.; Pfaffl, M.W.; Meyer, H.H.D.; Bauer, J.; Meyer, K.H.D. Expression of immune relevant genes in pigs under the influence of low doses of deoxynivalenol (DON). Mycotoxin Res. 2011, 27, 287–293. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol-induced IgA production and IgA nephropathy-aberrant mucosal immune response with systemic repercussions. Toxicol. Lett. 2003, 140, 287–295. [Google Scholar] [CrossRef]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Add. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. FeedSci. Technol. 2004, 114, 205–239. [Google Scholar]
- Ghareeb, K.; Awad, W.A.; Böhm, J. Ameliorative effect of a microbial feed additive on infectious bronchitis virus antibody titer and stress index in broiler chicks fed deoxynivalenol. Poult. Sci. 2012, 91, 800–807. [Google Scholar] [CrossRef]
- Dänicke, S.; Ueberschär, K.H.; Matthes, S.; Halle, I.; Valenta, H.; Flachowsky, G. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin contaminated maize on performance of hens and on carryover of zearalenone. Poult. Sci. 2002, 81, 1671–1680. [Google Scholar]
- Awad, W.A.; Ghareeb, K.; Chimidtseren, S.; Strasser, A.; Hess, M.; Böhm, J. Chronic Effects of Deoxynivalenol on Plasma Cytokines and Vaccine Response of Broiler Chickens. In Proceedings of the 34th Mykotoxin-Workshops der Ges. für Mykotoxin Forschung e.V., Braunschweig, Germany, 14–16 May 2012; p. 29.
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Hulan, H.W.; Zentek, J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult. Sci. 2004, 83, 1964–1972. [Google Scholar]
- Awad, W.A.; Razzazi-Fazeli, E.; Böhm, J.; Ghareeb, K.; Zentek, J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 2006, 85, 974–979. [Google Scholar]
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J. Anim. Nutr. Anim. Physiol. 2006, 90, 32–37. [Google Scholar] [CrossRef]
- Girgis, G.N.; Barta, J.R.; Brash, M.; Smith, T.K. Morphological changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis. 2010, 54, 67–73. [Google Scholar] [CrossRef]
- Romer Labs’ Guide to Mycotoxins. Mycotoxins—An Overview; Richard, J.L. (Ed.) Anytime Publishing Services: Leicestershire, UK, 2000; Volume 1, pp. 1–48.
- CAST, Council for Agricultural Science and Technology, Mycotoxins—Risks in Plant, Animal and Human Systems; (Task Force Report, No. 139); Council for Agricultural Science and Technology: Ames, IA, USA, 2003; Volume 10, pp. 1–191.
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microb. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health. B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Rotter, B.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol. J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar]
- Prelusky, D.B.; Hamilton, R.M.; Trenholm, H.L.; Miller, J.D. Tissue distribution and excretion of radioactivity following administration of 14C-labeled deoxynivalenol to White Leghorn hens. Fundam. Appl. Toxicol. 1986, 7, 635–645. [Google Scholar] [CrossRef]
- Gauvreau, H.C. Toxicokinetic, Tissue Residue, and Metabolic Studies of Deoxynivalenol (Vomitoxin) in Turkeys. Master thesis, Simon Fraser University, Vancouver, Canada, 2000. [Google Scholar]
- He, P.; Young, L.G.; Forsberg, C. Microbial transformation of deoxynivalenol (vomitoxin). Appl. Environ. Microbiol. 1992, 58, 3857–3863. [Google Scholar]
- Lun, A.K.; Moran, E.T., Jr.; Young, L.G.; McMillan, E.G. Absorption and elimination of an oral dose of 3H-deoxynivalenol in colostomized and intact chickens. Bull. Environ. Contam. Toxicol. 1989, 42, 919–925. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Dadak, A.; Gille, L.; Staniek, K.; Hess, M.; Böhm, J. Genotoxic effects of deoxynivalenol in broiler chickens fed with low protein diets. Poult. Sci. 2012, 91, 550–555. [Google Scholar]
- Dänicke, S.; Matthes, S.; Halle, I.; Ueberschar, K.H.; Döll, S.; Valenta, H. Effects of graded levels of Fusarium toxincontaminated wheat and of a detoxifying agent in broiler diets on performance, nutrient digestibility and blood chemical parameters. Br. Poult. Sci. 2003, 44, 113–126. [Google Scholar] [CrossRef]
- Xu, L.; Eicher, S.D.; Applegate, T.J. Effects of increasing dietary concentrations of corn naturally contaminated with deoxynivalenol on broiler and turkey poult performance and response to lipopolysaccharide. Poult. Sci. 2011, 90, 2766–2774. [Google Scholar] [CrossRef]
- Branton, S.L.; Deaton, J.W.; Hagler, W.M.; Maslin, W.R., Jr.; Hardin, J.M. Decreased egg production in commercial laying hens fed zearalenone- and deoxynivalenol-contaminated grain sorghum. Avian Dis. 1989, 33, 804–808. [Google Scholar] [CrossRef]
- Davis, G.S.; Anderson, K.E.; Parkhurst, C.R.; Rives, D.V.; Hagler, W.M. Mycotoxins and feed refusal by pekin ducks. J. Appl. Poult. Res. 1994, 3, 190–192. [Google Scholar]
- Dänicke, S.; Valenta, H.; Ueberschär, K.H.; Matthes, S. On the interactions between Fusarium toxin-contaminated wheat and non-starch-polysaccharide hydrolysing enzymes in turkey diets on performance, health and carry-over of deoxynivalenol and zearalenone. Br. Poult. Sci. 2007, 48, 39–48. [Google Scholar] [CrossRef]
- Kubena, L.F.; Edrington, T.S.; Harvey, R.B.; Phillips, T.D.; Sarr, A.B.; Rottinghaus, G.E. Individual and combined effects of fumonisin B1 present in Fusarium moniliforme culture material and diacetoxyscirpenol or ochratoxin A in turkey poults. Poult. Sci. 1997, 76, 256–264. [Google Scholar]
- Harvey, R.B.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Casper, H.H.; Buckley, S.A. Moniliformin from Fusarium fujikuroi culture material and deoxynivalenol from naturally contaminated wheat incorporated into diets of broiler chicks. Avian Dis. 1997, 41, 957–963. [Google Scholar] [CrossRef]
- Leitgeb, R.; Lew, H.; Wetscherek, W.; Böhm, J.; Quinz, A. Influence of fusariotoxins on growing and slaughtering performance of broilers. Aust. J. Agric. Res. 1999, 50, 57–66. [Google Scholar]
- Swamy, H.V.L.N.; Smith, T.K.; Cotter, P.F.; Boermans, H.J.; Seftons, A.E. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on production and metabolism in broilers. Poult. Sci. 2002, 81, 966–975. [Google Scholar]
- Li, Y.D.; Verstegen, M.W.A.; Gerrits, W.J.J. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 2003, 16, 223–239. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, M.; Twarużek, M.; Grajewski, J.; Kosicki, R.; Böhm, J.; Zentek, J. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int. J. Mol. Sci. 2011, 12, 7996–8012. [Google Scholar] [CrossRef]
- Awad, W.A.; Vahjen, W.; Aschenbach, J.R.; Zentek, J. A diet naturally contaminated with the Fusarium mycotoxin deoxynivalenol (DON) downregulates gene expression of glucose transporters in the intestine of broiler chickens. Livest. Sci. 2011, 140, 72–79. [Google Scholar] [CrossRef]
- Keshavarz, K. Corn contaminated with deoxynivalenol: Effects on Performance of poultry. J. Appl. Poult. Res. 1993, 2, 43–50. [Google Scholar]
- Awad, W.A.; Rehman, H.; Böhm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of luminal deoxynivalenol and L-proline on electrophysiological parameters in the jejunums of laying hens. Poult. Sci. 2005, 84, 928–932. [Google Scholar]
- Bindslev, N.; Hirayama, B.A.; Wright, E.M. Na/D-glucose cotransport and SGLT1 expression in hen colon correlates with dietary Na+. Comp. Biochem. Physiol. A 1997, 118, 219–227. [Google Scholar] [CrossRef]
- Morris, C.M.; Li, Y.C.; Ledoux, D.R.; Bermudez, A.J.; Rottinghaus, G.E. The individual and combined effects of feeding moniliformin, supplied by Fusarium fujikuroi culture material, and deoxynivalenol in young turkey poults. Poult. Sci. 1999, 78, 1110–1115. [Google Scholar]
- Feinberg, B.; Mclaughlin, C.S. Biochemical Mechanism of Action of Trichothecene Mycotoxins. In Trichothecene Mycotoxicosis: Pathophysiologic Effects; Beasley, V.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume I, pp. 27–35. [Google Scholar]
- Dänicke, S.; Ueberschär, K.H.; Valenta, H.; Matthes, S.; Matthäus, K.; Halle, I. Effects of graded levels of Fusarium-toxin-contaminated wheat in Pekin duck diets on performance, health and metabolism of deoxynivalenol and zearalenone. Br. Poult. Sci. 2004, 45, 264–272. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Chang, M.H.; Lin, Y.A.; Wu, J.F; Chen, B.J. Effects of deoxynivalenol and degradation enzyme on growth performance and immune responses in mule ducks. J. Anim. Feed Sci. 2004, 13, 275–287. [Google Scholar]
- Kubena, L.F.; Swanson, S.P.; Harvey, R.B.; Fletcher, O.J.; Rowe, L.D.; Phillips, T.D. Effects of feeding deoxynivalenol (vomitoxin)-contaminated wheat to growing chicks. Poult. Sci. 1985, 64, 1649–1655. [Google Scholar] [CrossRef]
- Lun, A.K.; Young, L.G.; Moran, J.E.T.; Hunter, D.B.; Rodriguez, J.P. Effects of feeding hens a high level of vomitoxin-contaminated corn on performance and tissues residues. Poult. Sci. 1986, 65, 1095–1099. [Google Scholar] [CrossRef]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar]
- Hughes, R.J. An integrated approach to understanding gut function and gut health of chickens. Asia Pac. J. Clin. Nutri. 2005, 14, S27. [Google Scholar]
- Bouhet, S.; Oswald, I.P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 2005, 108, 199–209. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar]
- Gunther, A.; van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.; Eeckhout, M.; de Saeger, S.; et al. Deoxynivalenol Predisposes for Necrotic Enteritis by Affecting the intestinal Barrier in Broilers. In Proceedings of the International Poultry Scientific Forum, Georgia World Congress Center, Atlanta, GA, USA, 28–29 January 2013; pp. 9–10.
- Pestka, J.J.; Bondy, G.S. Alteration of immune function following dietary mycotoxin exposure. Can. J. Physiol. Pharmacol. 1990, 68, 1009–1016. [Google Scholar] [CrossRef]
- Dong, W.; Azcona Olivera, J.I.; Brooks, K.H.; Linz, J.E.; Pestka, J.J. Elevated gene expression and production of interleukins 2, 4, 5 and 6 during exposure to vomitoxin (deoxynivalenol) and cycloheximide in the EL-4 thymoma. Toxicol. Appl. Pharmacol. 1994, 127, 282–290. [Google Scholar] [CrossRef]
- Warner, R.L.; Brooks, K.; Pestka, J.J. in vitro effects of vomitoxin (Deoxynivalenol) on lymphocyte function: Enhanced interleukin production and help for IgA secretion. Food Chem. Toxicol. 1994, 32, 617–625. [Google Scholar] [CrossRef]
- Harvey, R.B.; Kubena, L.F.; Huff, W.E.; Elissalde, M.H.; Phillips, T.D. Heamatological and immunological toxicity of deoxynivalenol-contaminated diets to growing chickens. Bull. Environ. Contam. Toxicol. 1991, 40, 410–416. [Google Scholar]
- Yunus, A.W.; Ghareeb, K.; Twaruzek, M.; Grajewski, J.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Effects on bird performance and response to common vaccines. Poult. Sci. 2012, 91, 844–851. [Google Scholar] [CrossRef]
- Yegani, M.; Smith, T.K.; Leeson, S.; Boermans, H.J. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on performance and metabolism of broiler breeders. Poult. Sci. 2006, 85, 1541–1549. [Google Scholar]
- Pestka, J.J.; Moorman, M.A.; Warner, R.L. Dysregulation of IgA production and IgA nephropathy induced by the trichothecene vomitoxin. Food Chem. Toxicol. 1989, 27, 361–368. [Google Scholar] [CrossRef]
- Dong, W.; Sell, J.E.; Pestka, J.J. Quantitative assessment of mesangial immunoglobulin A (IgA) accumulation, elevated circulating IgA immune complexes, and hematuria during vomitoxin-induced IgA nephropathy. Fundam. Appl. Toxicol. 1991, 17, 197–207. [Google Scholar] [CrossRef]
- Dong, W.; Pestka, J.J. Persistent dysregulation of IgA production and IgA nephropathy in the B6C3F1 mouse following withdrawal of dietary vomitoxin (deoxynivalenol). Fundam. Appl. Toxicol. 1993, 20, 38–47. [Google Scholar] [CrossRef]
- Bondy, G.S.; Pestka, J.J. Dietary exposure to the trichothecene vomitoxin (Deoxynivalenol) stimulates terminal differentiation of Peyer’s patch B cells to IgA secreting plasma cells. Toxicol. Appl. Pharmacol. 1991, 108, 520–530. [Google Scholar] [CrossRef]
- Rasooly, L.; Pestka, J.J. Polyclonal autoreactive IgA increase and mesangial deposition during vomitoxin induced IgA nephropahty in the BALB/c mouse. Food Chem. Toxicol. 1994, 32, 329–336. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Smith, T.K.; Boermans, H.J.; Woodward, B. Effects of feed-borne Fusarium mycotoxins on hematology and immunology of laying hens. Poult. Sci. 2005, 84, 1841–1850. [Google Scholar]
- Li, Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Jiang, S.Z.; Wu, Y.B. Effects of feed-borne Fusarium mycotoxins with or without yeast cell wall adsorbent on organ weight, serum biochemistry, and immunological parameters of broiler chickens. Poult. Sci. 2012, 91, 2487–2495. [Google Scholar] [CrossRef]
- Pestka, J.J.; Yan, D.; King, L.E. Flow cytometric analysis of the effects of in vitro exposure to vomitoxin (deoxynivalenol) on apoptosis in murine T B and IgA+ cells. Food Chem. Toxicol. 1994, 32, 1125–1136. [Google Scholar] [CrossRef]
- Islam, Z.; Nagase, M.; Ota, A.; Ueda, S.; Yoshizawa, T.; Sakato, N. Structure-function relationship of T-2 toxin and its metabolites in inducing thymic apoptosis in vivo in mice. Biosci. Biotechnol. Biochem. 1998, 62, 1492–1497. [Google Scholar] [CrossRef]
- Frankic, T.; Pajk, T.; Rezar, V.; Levart, A.; Salobir, J. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem. Toxicol. 2006, 44, 1838–1844. [Google Scholar] [CrossRef]
- Yan, D.; Zhou, H.R.; Brooks, K.H.; Pestka, J.J. Potential role for IL-5 and IL-6 in enhanced IgA secretion by Peyer’s patch cells isolated from mice acutely exposed to vomitoxin. Toxicology 1997, 122, 145–158. [Google Scholar] [CrossRef]
- Ouyang, Y.L.; Azcona Olivera, J.I.; Murtha, J.M.; Pestka, J.J. Vomitoxin mediated IL2, IL4 and IL5 superinduction in murine CD4+ T cells stimulated with phorbol ester calcium ionophore: Relation to kinetics of poliferation. Toxicol. Appl. Pharmacol. 1996, 138, 324–334. [Google Scholar] [CrossRef]
- Miller, K.; Atkinson, H.A. The in vitro effects of trichothecenes on the immune system. Arch. Toxicol. 1987, 11, 321–324. [Google Scholar]
- Islam, Z.; Gray, J.S.; Pestka, J.J. p38 mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicol. Appl. Pharmacol. 2005, 213, 235–244. [Google Scholar] [CrossRef]
- Wong, S.S.; Zhou, H.R.; Marin-Martinez, M.L.; Brooks, K.; Pestka, J.J. Modulation of IL-1, IL-6 and TNF- secretion and mRNA expression by the trichothecene vomitoxin in the RAW 264.7 murine macrophage cell line. Food Chem. Toxicol. 1998, 36, 409–419. [Google Scholar] [CrossRef]
- Azcona-Olivera, J.I.; Ouyang, Y.L.; Warner, R.L.; Linz, J.E.; Pestka, J.J. Effects on vomitoxin (Deoxynivalenol) and cyclohexamide on IL-2, 4, 5 and 6 secretion and mRNA levels in murine CD4+ cells. Food Chem. Toxicol. 1995, 33, 433–441. [Google Scholar] [CrossRef]
- Zhou, H.R.; Yan, D.; Pestka, J.J. Differential cytokine mRNA expression in mice after oral exposure to the trichothecene vomitoxin (Deoxynivalenol): Dose response and time course. Toxicol. Appl. Pharmacol. 1997, 144, 294–305. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Jiang, S.Z.; Wu, Y.B. Effects of feed-borne Fusarium mycotoxins with or without yeast cell wall adsorbent on organ weight, serum biochemistry, and immunological parameters of broiler chickens. Poult. Sci. 2012, 91, 2487–2495. [Google Scholar] [CrossRef]
- Girgis, G.N.; Shayan, S.; Barta, J.R.; Boermans, H.J.; Smith, T.K. Immunomodulatory effects of feed-borne Fusarium mycotoxins in chickens infected with Coccidia. Exp. Biol. Med. 2008, 233, 1411–1420. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Awad, W.; Ghareeb, K.; Böhm, J.; Zentek, J. The Toxicological Impacts of the Fusarium Mycotoxin, Deoxynivalenol, in Poultry Flocks with Special Reference to Immunotoxicity. Toxins 2013, 5, 912-925. https://doi.org/10.3390/toxins5050912
Awad W, Ghareeb K, Böhm J, Zentek J. The Toxicological Impacts of the Fusarium Mycotoxin, Deoxynivalenol, in Poultry Flocks with Special Reference to Immunotoxicity. Toxins. 2013; 5(5):912-925. https://doi.org/10.3390/toxins5050912
Chicago/Turabian StyleAwad, Wageha, Khaled Ghareeb, Josef Böhm, and Jürgen Zentek. 2013. "The Toxicological Impacts of the Fusarium Mycotoxin, Deoxynivalenol, in Poultry Flocks with Special Reference to Immunotoxicity" Toxins 5, no. 5: 912-925. https://doi.org/10.3390/toxins5050912




