Structure, Function, and Biology of the Enterococcus faecalis Cytolysin
Abstract
:1. Introduction: The Enterococci as Emergent Hospital Pathogens
2. Cytolysin and Toxicity of Enterococcal Infections
| Setting | Effect of Cytolysin | Reference |
|---|---|---|
| Human bacteremia | Cytolysin makes infection five times more acutely lethal | [22] |
| Rabbit endophthalmitis | Cytolysin makes infection acutely destructive to retina and other ocular structures, and refractory to antibiotic treatment | [46,47,49] |
| Mouse intraperitoneal infection | Cytolysin makes infection approximately one hundred times more acutely lethal | [42,50] |
| Rabbit endocarditis | Cytolysin makes infection acutely lethal in synergy with aggregation substance | [45] |
| C. elegans ingestion | Cytolysin makes infection acutely lethal following ingestion | [48] |
3. Cytolysin Structure and Function
3.1. Overview of the Cytolysin
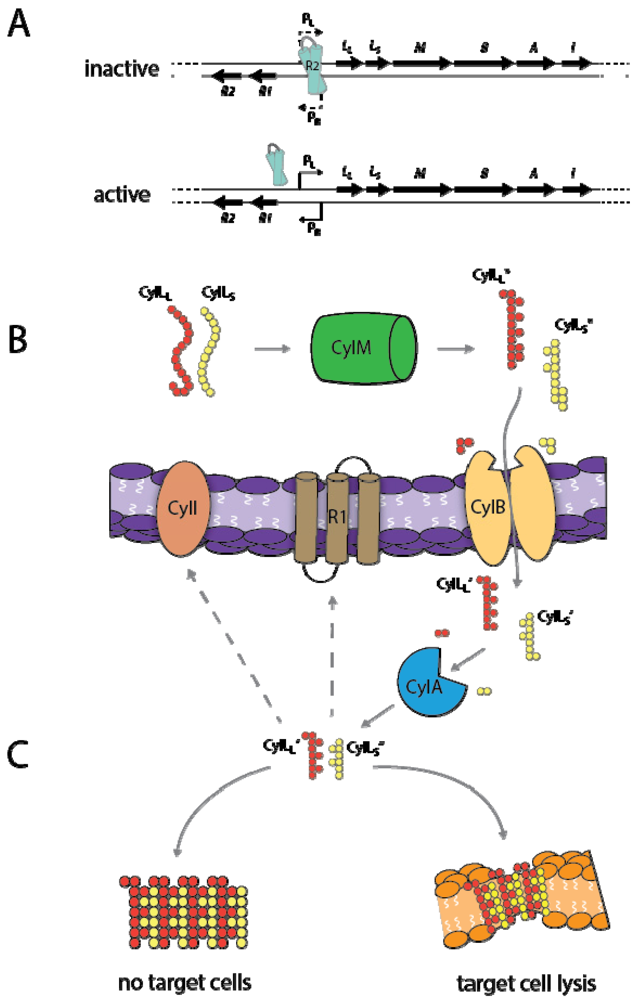
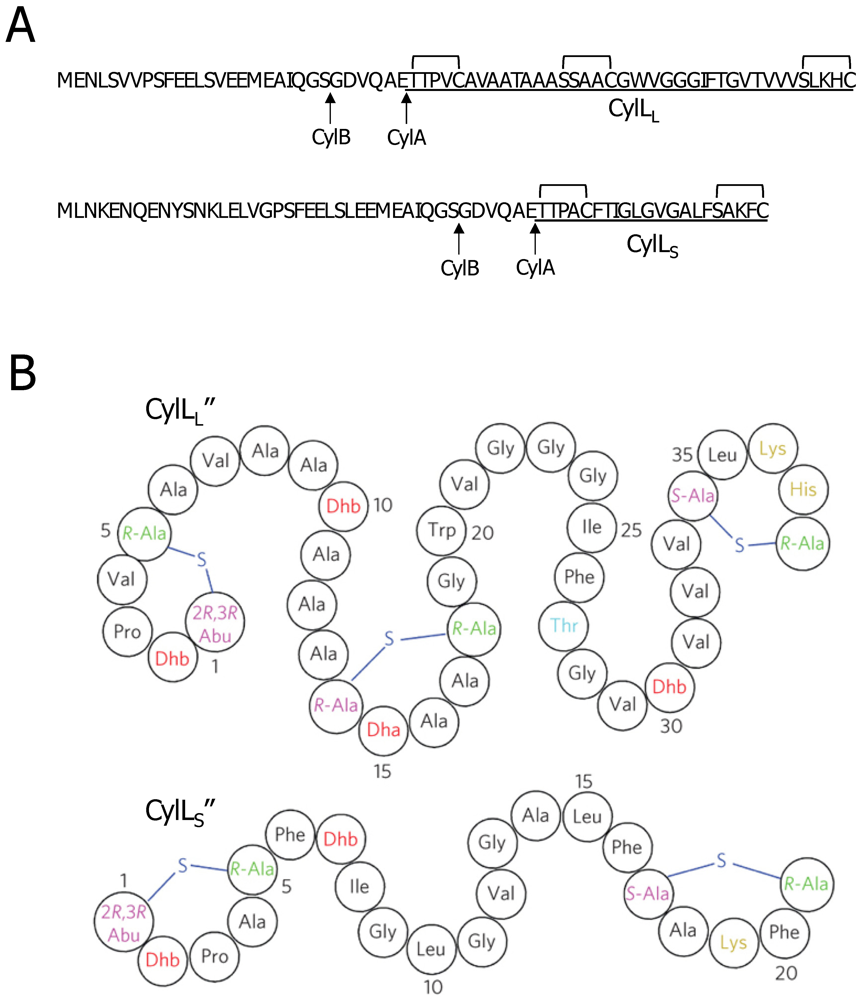
3.2. Cytolysin Structural and Molecular Properties
3.3. Cytolysin Regulation
3.4. Toxin Mechanism of Action
3.5. Biological Role of Cytolysin
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Gilmore, M.S.; Lebreton, F.; van Schaik, W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr. Opin. Microbiol. 2013, 16, 10–16. [Google Scholar]
- Mundt, J.O. Occurrence of enterococci in animals in a wild environment. Appl. Microbiol. 1963, 11, 136–140. [Google Scholar]
- Lawley, T.D.; Walker, A.W. Intestinal colonization resistance. Immunology 2013, 138, 1–11. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Jett, B.D.; Huycke, M.M.; Gilmore, M.S. Virulence of enterococci. Clin. Microbiol. Rev. 1994, 7, 462–478. [Google Scholar]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Clements, C.V.; Rollins, D.; Rodrigues, J.L.; Hooper, L.V. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc. Natl. Acad. Sci. USA 2012, 109, 17621–17626. [Google Scholar]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. Nhsn annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial infections in combined medical-surgical intensive care units in the united states. Infect. Control Hosp. Epidemiol. 2000, 21, 510–515. [Google Scholar]
- Maki, D.G.; Agger, W.A. Enterococcal bacteremia: Clinical features, the risk of endocarditis, and management. Medicine 1988, 67, 248–269. [Google Scholar] [CrossRef]
- Huycke, M.M.; Sahm, D.F.; Gilmore, M.S. Multiple-drug resistant enterococci: The nature of the problem and an agenda for the future. Emerg. Infect. Dis. 1998, 4, 239–249. [Google Scholar] [CrossRef]
- Murray, B.E. The life and times of enterococcus. Clin. Microbiol. Rev. 1990, 3, 46. [Google Scholar]
- Paulsen, I.T.; Banerjei, L.; Myers, G.S.; Nelson, K.E.; Seshadri, R.; Read, T.D.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; Heidelberg, J.F.; et al. Role of mobile DNA in the evolution of vancomycin-resistant enterococcus faecalis. Science 2003, 299, 2071–2074. [Google Scholar] [CrossRef]
- Dunny, G.M.; Leonard, B.A.; Hedberg, P.J. Pheromone-inducible conjugation in enterococcus faecalis: Interbacterial and host-parasite chemical communication. J. Bacteriol. 1995, 177, 871–876. [Google Scholar]
- Clewell, D.B.; Gawron-Burke, C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu. Rev. Microbiol. 1986, 40, 635–659. [Google Scholar] [CrossRef]
- LeBlanc, D.J.; Lee, L.N.; Clewell, D.B.; Behnke, D. Broad geographical distribution of a cytotoxin gene mediating beta-hemolysis and bacteriocin activity among streptococcus faecalis strains. Infect. Immun. 1983, 40, 1015–1022. [Google Scholar]
- Dunny, G.M.; Leonard, B.A. Cell-cell communication in gram-positive bacteria. Annu. Rev. Microbiol. 1997, 51, 527–564. [Google Scholar] [CrossRef]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef]
- Toala, P.; McDonald, A.; Wilcox, C.; Finland, M. Susceptibility of group d streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am. J. Med. Sci. 1969, 258, 416–430. [Google Scholar]
- Lawley, T.D.; Clare, S.; Walker, A.W.; Stares, M.D.; Connor, T.R.; Raisen, C.; Goulding, D.; Rad, R.; Schreiber, F.; Brandt, C.; et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing clostridium difficile disease in mice. PLoS Pathog. 2012, 8, e1002995. [Google Scholar] [CrossRef] [Green Version]
- Clewell, D.B.; Franke, A.E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin balpha in a strain streptococcus pyogenens. Antimicrob. Agents Chemother. 1974, 5, 534–537. [Google Scholar] [CrossRef]
- Huycke, M.M.; Spiegel, C.A.; Gilmore, M.S. Bacteremia caused by hemolytic, high-level gentamicin-resistant enterococcus faecalis. Antimicrob. Agents Chemother. 1991, 35, 1626–1634. [Google Scholar] [CrossRef]
- Sahm, D.F.; Kissinger, J.; Gilmore, M.S.; Murray, P.R.; Mulder, R.; Solliday, J.; Clarke, B. In vitro susceptibility studies of vancomycin-resistant enterococcus faecalis. Antimicrob. Agents Chemother. 1989, 33, 1588–1591. [Google Scholar] [CrossRef]
- Mutnick, A.H.; Biedenbach, D.J.; Jones, R.N. Geographic variations and trends in antimicrobial resistance among enterococcus faecalis and enterococcus faecium in the sentry antimicrobial surveillance program (1997–2000). Diagn. Microbiol. Infect. Dis. 2003, 46, 63–68. [Google Scholar] [CrossRef]
- De Fatima Silva Lopes, M.; Ribeiro, T.; Abrantes, M.; Figueiredo Marques, J.J.; Tenreiro, R.; Crespo, M.T. Antimicrobial resistance profiles of dairy and clinical isolates and type strains of enterococci. Int. J. Food Microbiol. 2005, 103, 191–198. [Google Scholar] [CrossRef]
- McBride, S.M.; Fischetti, V.A.; Leblanc, D.J.; Moellering, R.C., Jr.; Gilmore, M.S. Genetic diversity among enterococcus faecalis. PLoS One 2007, 2, e582. [Google Scholar]
- Thiercelin, M.E. Sur un diplocoque saprophyte de l’intestin susceptible de devenir pathogen. C R Soc. Biol. 1899, 5, 269–271. [Google Scholar]
- Niven, C.F.; Sherman, J.M. Nutrition of the enterococci. J. Bacteriol. 1944, 47, 335–342. [Google Scholar]
- Thiercelin, M.E. Morphology and mode of reproduction of the ‘microbe enterocoque’. C. R. Soc. Biol. 1899, 11, 551–553. [Google Scholar]
- Maccallum, W.G.; Hastings, T.W. A case of acute endocarditis caused by micrococcus zymogenes (nov. Spec.), with a description of the microorganism. J. Exp. Med. 1899, 4, 521–534. [Google Scholar] [CrossRef]
- Todd, E.W. A comparative serological study of streptolysins dervived from human and from animal infections, with notes on pneumococcal haemolysin, tetanolysin and staphylococcus toxin. J. Pathol. Bateriol. 1934, 39, 299–321. [Google Scholar] [CrossRef]
- Kobayashi, R. Studies concerning hemolytic streptococci: Typing of human hemolytic streptococci and their relation to diseases and their distribution on mucous membranes. Kitasato Arch. Exp. Med. 1940, 17, 218–241. [Google Scholar]
- Brock, T.D.; Peacher, B.; Pierson, D. Survey of the bacteriocines of enterococci. J. Bacteriol. 1963, 86, 702–707. [Google Scholar]
- Roelofsen, B.; de Gier, J.; van, D. Binding of lipids in the red cell membrane. J. Cell. Physiol. 1964, 63, 233–243. [Google Scholar] [CrossRef]
- Basinger, S.F.; Jackson, R.W. Bacteriocin (hemolysin) of streptococcus zymogenes. J. Bacteriol. 1968, 96, 1895–1902. [Google Scholar]
- Elsner, H.A.; Sobottka, I.; Mack, D.; Claussen, M.; Laufs, R.; Wirth, R. Virulence factors of enterococcus faecalis and enterococcus faecium blood culture isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 39–42. [Google Scholar] [CrossRef]
- Karen Carniol, M.S.G. Enterococcus faecalis cytolysin toxin. In The Comprehensive Sourcebook of Bacterial Protein Toxins, 3rd; Joseph, E., Alouf, M.R.P., Eds.; Academic Press: Burlington, MA, USA, 2006; pp. 717–727. [Google Scholar]
- Sherwood, N.P.; Russell, B.E. New antibiotic substances produced by beta hemolytic streptococci. J. Infect. Dis. 1949, 84, 88–91. [Google Scholar] [CrossRef]
- Brock, T.D.; Davie, J.M. Probable identity of a group d hemolysin with a bacteriocine. J. Bacteriol. 1963, 86, 708–712. [Google Scholar]
- Stark, J.M. Antibiotic activity of haemolytic enterococci. Lancet 1960, 1, 733–734. [Google Scholar] [CrossRef]
- Gilmore, M.S.; Segarra, R.A.; Booth, M.C. An hlyb-type function is required for expression of the enterococcus faecalis hemolysin/bacteriocin. Infect. Immun. 1990, 58, 3914–3923. [Google Scholar]
- Ike, Y.; Hashimoto, H.; Clewell, D.B. Hemolysin of streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect. Immun. 1984, 45, 528–530. [Google Scholar]
- Ike, Y.; Clewell, D.B. Evidence that the hemolysin/bacteriocin phenotype of enterococcus faecalis subsp. Zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J. Bacteriol. 1992, 174, 8172–8177. [Google Scholar]
- Miyazaki, S.; Ohno, A.; Kobayashi, I.; Uji, T.; Yamaguchi, K.; Goto, S. Cytotoxic effect of hemolytic culture supernatant from enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol. Immunol. 1993, 37, 265–270. [Google Scholar]
- Chow, J.W.; Thal, L.A.; Perri, M.B.; Vazquez, J.A.; Donabedian, S.M.; Clewell, D.B.; Zervos, M.J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 1993, 37, 2474–2477. [Google Scholar] [CrossRef]
- Jett, B.D.; Jensen, H.G.; Nordquist, R.E.; Gilmore, M.S. Contribution of the pad1-encoded cytolysin to the severity of experimental enterococcus faecalis endophthalmitis. Infect. Immun. 1992, 60, 2445–2452. [Google Scholar]
- Stevens, S.X.; Jensen, H.G.; Jett, B.D.; Gilmore, M.S. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental enterococcus faecalis endophthalmitis. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1650–1656. [Google Scholar]
- Garsin, D.A.; Sifri, C.D.; Mylonakis, E.; Qin, X.; Singh, K.V.; Murray, B.E.; Calderwood, S.B.; Ausubel, F.M. A simple model host for identifying gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 2001, 98, 10892–10897. [Google Scholar] [CrossRef]
- Jett, B.D.; Jensen, H.G.; Atkuri, R.V.; Gilmore, M.S. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing enterococcus faecalis strains. Investig. Ophthalmol. Vis. Sci. 1995, 36, 9–15. [Google Scholar]
- Singh, K.V.; Qin, X.; Weinstock, G.M.; Murray, B.E. Generation and testing of mutants of enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 1998, 178, 1416–1420. [Google Scholar]
- Booth, M.C.; Hatter, K.L.; Miller, D.; Davis, J.; Kowalski, R.; Parke, D.W.; Chodosh, J.; Jett, B.D.; Callegan, M.C.; Penland, R.; et al. Molecular epidemiology of staphylococcus aureus and enterococcus faecalis in endophthalmitis. Infect. Immun. 1998, 66, 356–360. [Google Scholar]
- Ike, Y.; Hashimoto, H.; Clewell, D.B. High incidence of hemolysin production by enterococcus (streptococcus) faecalis strains associated with human parenteral infections. J. Clin. Microbiol. 1987, 25, 1524–1528. [Google Scholar]
- Semedo, T.; Almeida Santos, M.; Martins, P.; Silva Lopes, M.F.; Figueiredo Marques, J.J.; Tenreiro, R.; Barreto Crespo, M.T. Comparative study using type strains and clinical and food isolates to examine hemolytic activity and occurrence of the cyl operon in enterococci. J. Clin. Microbiol. 2003, 41, 2569–2576. [Google Scholar] [CrossRef]
- Solheim, M.; Aakra, A.; Snipen, L.G.; Brede, D.A.; Nes, I.F. Comparative genomics of enterococcus faecalis from healthy norwegian infants. BMC Genomics 2009, 10, 194. [Google Scholar]
- Park, S.Y.; Kim, K.M.; Lee, J.H.; Seo, S.J.; Lee, I.H. Extracellular gelatinase of enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect. Immun. 2007, 75, 1861–1869. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Thomas, V.C.; Fleming, S.D.; Hancock, L.E. Enterococcus faecalis capsular polysaccharide serotypes c and d and their contributions to host innate immune evasion. Infect. Immun. 2009, 77, 5551–5557. [Google Scholar]
- Nes, I.F.; Diep, D.B.; Holo, H. Bacteriocin diversity in streptococcus and enterococcus. J. Bacteriol. 2007, 189, 1189–1198. [Google Scholar] [CrossRef]
- Dunny, G.M.; Clewell, D.B. Transmissible toxin (hemolysin) plasmid in streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J. Bacteriol. 1975, 124, 784–790. [Google Scholar]
- Sussmuth, S.D.; Muscholl-Silberhorn, A.; Wirth, R.; Susa, M.; Marre, R.; Rozdzinski, E. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 2000, 68, 4900–4906. [Google Scholar] [CrossRef]
- Shankar, N.; Baghdayan, A.S.; Gilmore, M.S. Modulation of virulence within a pathogenicity island in vancomycin-resistant enterococcus faecalis. Nature 2002, 417, 746–750. [Google Scholar] [CrossRef]
- Clewell, D.B.; Tomich, P.K.; Gawron-Burke, M.C.; Franke, A.E.; Yagi, Y.; An, F.Y. Mapping of streptococcus faecalis plasmids pad1 and pad2 and studies relating to transposition of tn917. J. Bacteriol. 1982, 152, 1220–1230. [Google Scholar]
- Ike, Y.; Clewell, D.B.; Segarra, R.A.; Gilmore, M.S. Genetic analysis of the pad1 hemolysin/bacteriocin determinant in enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J. Bacteriol. 1990, 172, 155–163. [Google Scholar]
- Clewell, D.B. Bacterial sex pheromone-induced plasmid transfer. Cell 1993, 73, 9–12. [Google Scholar] [CrossRef]
- Segarra, R.A.; Booth, M.C.; Morales, D.A.; Huycke, M.M.; Gilmore, M.S. Molecular characterization of the enterococcus faecalis cytolysin activator. Infect. Immun. 1991, 59, 1239–1246. [Google Scholar]
- Gilmore, M.S.; Segarra, R.A.; Booth, M.C.; Bogie, C.P.; Hall, L.R.; Clewell, D.B. Genetic structure of the enterococcus faecalis plasmid pad1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J. Bacteriol. 1994, 176, 7335–7344. [Google Scholar]
- Coburn, P.S.; Hancock, L.E.; Booth, M.C.; Gilmore, M.S. A novel means of self-protection, unrelated to toxin activation, confers immunity to the bactericidal effects of the enterococcus faecalis cytolysin. Infect. Immun. 1999, 67, 3339–3347. [Google Scholar]
- Haas, W.; Shepard, B.D.; Gilmore, M.S. Two-component regulator of enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 2002, 415, 84–87. [Google Scholar]
- Rumpel, S.; Razeto, A.; Pillar, C.M.; Vijayan, V.; Taylor, A.; Giller, K.; Gilmore, M.S.; Becker, S.; Zweckstetter, M. Structure and DNA-binding properties of the cytolysin regulator cylr2 from enterococcus faecalis. EMBO J. 2004, 23, 3632–3642. [Google Scholar] [CrossRef]
- Booth, M.C.; Bogie, C.P.; Sahl, H.G.; Siezen, R.J.; Hatter, K.L.; Gilmore, M.S. Structural analysis and proteolytic activation of enterococcus faecalis cytolysin, a novel lantibiotic. Mol. Microbiol. 1996, 21, 1175–1184. [Google Scholar]
- Willey, J.M.; van der Donk, W.A. Lantibiotics: Peptides of diverse structure and function. Annu. Rev. Microbiol. 2007, 61, 477–501. [Google Scholar] [CrossRef]
- Coburn, P.S.; Pillar, C.M.; Jett, B.D.; Haas, W.; Gilmore, M.S. Enterococcus faecalis senses target cells and in response expresses cytolysin. Science 2004, 306, 2270–2272. [Google Scholar] [CrossRef]
- Sahl, H.G.; Jack, R.W.; Bierbaum, G. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur. J. Biochem. 1995, 230, 827–853. [Google Scholar] [CrossRef]
- Coburn, P.S.; Gilmore, M.S. The enterococcus faecalis cytolysin: A novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 2003, 5, 661–669. [Google Scholar] [CrossRef]
- Dougherty, B.A.; Hill, C.; Weidman, J.F.; Richardson, D.R.; Venter, J.C.; Ross, R.P. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pmrc01 from lactococcus lactis dpc3147. Mol. Microbiol. 1998, 29, 1029–1038. [Google Scholar] [CrossRef]
- McClerren, A.L.; Cooper, L.E.; Quan, C.; Thomas, P.M.; Kelleher, N.L.; van der Donk, W.A. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. USA 2006, 103, 17243–17248. [Google Scholar]
- Tang, W.; van der Donk, W.A. The sequence of the enterococcal cytolysin imparts unusual lanthionine stereochemistry. Nat. Chem. Biol. 2013, 9, 157–159. [Google Scholar] [CrossRef]
- Thanabalu, T.; Koronakis, E.; Hughes, C.; Koronakis, V. Substrate-induced assembly of a contiguous channel for protein export from e.Coli: Reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998, 17, 6487–6496. [Google Scholar] [CrossRef]
- Khosla, C.; Tang, Y.; Chen, A.Y.; Schnarr, N.A.; Cane, D.E. Structure and mechanism of the 6-deoxyerythronolide b synthase. Annu. Rev. Biochem. 2007, 76, 195–221. [Google Scholar]
- Kiesau, P.; Eikmanns, U.; Gutowski-Eckel, Z.; Weber, S.; Hammelmann, M.; Entian, K.D. Evidence for a multimeric subtilin synthetase complex. J. Bacteriol. 1997, 179, 1475–1481. [Google Scholar]
- Shankar, N.; Coburn, P.; Pillar, C.; Haas, W.; Gilmore, M. Enterococcal cytolysin: Activities and association with other virulence traits in a pathogenicity island. Int. J. Med. Microbiol. 2004, 293, 609–618. [Google Scholar] [CrossRef]
- Croucher, N.J.; Fookes, M.C.; Perkins, T.T.; Turner, D.J.; Marguerat, S.B.; Keane, T.; Quail, M.A.; He, M.; Assefa, S.; Bahler, J.; et al. A simple method for directional transcriptome sequencing using illumina technology. Nucleic Acids Res. 2009, 37, e148. [Google Scholar] [CrossRef]
- Perraud, A.L.; Weiss, V.; Gross, R. Signalling pathways in two-component phosphorelay systems. Trends Microbiol. 1999, 7, 115–120. [Google Scholar] [CrossRef]
- Roux, A.; Payne, S.M.; Gilmore, M.S. Microbial telesensing: Probing the environment for friends, foes, and food. Cell Host Microbe 2009, 6, 115–124. [Google Scholar] [CrossRef]
- Leonard, B.A.; Podbielski, A.; Hedberg, P.J.; Dunny, G.M. Enterococcus faecalis pheromone binding protein, prgz, recruits a chromosomal oligopeptide permease system to import sex pheromone ccf10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 1996, 93, 260–264. [Google Scholar]
- Van Den Hooven, H.W.; Doeland, C.C.; van de Kamp, M.; Konings, R.N.; Hilbers, C.W.; van de ven, F.J. Three-dimensional structure of the lantibiotic nisin in the presence of membrane-mimetic micelles of dodecylphosphocholine and of sodium dodecylsulphate. Eur. J. Biochem. 1996, 235, 382–393. [Google Scholar]
- Islam, M.R.; Nagao, J.; Zendo, T.; Sonomoto, K. Antimicrobial mechanism of lantibiotics. Biochem. Soc. Trans. 2012, 40, 1528–1533. [Google Scholar] [CrossRef]
- Van Heusden, H.E.; de Kruijff, B.; Breukink, E. Lipid ii induces a transmembrane orientation of the pore-forming peptide lantibiotic nisin. Biochemistry 2002, 41, 12171–12178. [Google Scholar] [CrossRef]
- Hasper, H.E.; de Kruijff, B.; Breukink, E. Assembly and stability of nisin-lipid ii pores. Biochemistry 2004, 43, 11567–11575. [Google Scholar] [CrossRef]
- Wiedemann, I.; Bottiger, T.; Bonelli, R.R.; Wiese, A.; Hagge, S.O.; Gutsmann, T.; Seydel, U.; Deegan, L.; Hill, C.; Ross, P.; et al. The mode of action of the lantibiotic lacticin 3147--a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid ii. Mol. Microbiol. 2006, 61, 285–296. [Google Scholar] [CrossRef]
- Cox, C.R.; Coburn, P.S.; Gilmore, M.S. Enterococcal cytolysin: A novel two component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr. Protein Pept. Sci. 2005, 6, 77–84. [Google Scholar] [CrossRef]
- Wiedemann, I.; Benz, R.; Sahl, H.G. Lipid ii-mediated pore formation by the peptide antibiotic nisin: A black lipid membrane study. J. Bacteriol. 2004, 186, 3259–3261. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M. Mode of action of lipid ii-targeting lantibiotics. Int. J. Food Microbiol. 2005, 101, 201–216. [Google Scholar] [CrossRef]
- Stein, T.; Heinzmann, S.; Solovieva, I.; Entian, K.D. Function of lactococcus lactis nisin immunity genes nisi and nisfeg after coordinated expression in the surrogate host bacillus subtilis. J. Biol. Chem. 2003, 278, 89–94. [Google Scholar]
- Stein, T.; Heinzmann, S.; Dusterhus, S.; Borchert, S.; Entian, K.D. Expression and functional analysis of the subtilin immunity genes spaifeg in the subtilin-sensitive host bacillus subtilis mo1099. J. Bacteriol. 2005, 187, 822–828. [Google Scholar]
- Consortium, H.M.P. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Sudakaran, S.; Salem, H.; Kost, C.; Kaltenpoth, M. Geographical and ecological stability of the symbiotic mid-gut microbiota in european firebugs, pyrrhocoris apterus (hemiptera, pyrrhocoridae). Mol. Ecol. 2012, 21, 6134–6151. [Google Scholar] [CrossRef]
- Kautz, S.; Rubin, B.E.; Russell, J.A.; Moreau, C.S. Surveying the microbiome of ants: Comparing 454 pyrosequencing with traditional methods to uncover bacterial diversity. Appl. Environ. Microbiol. 2013, 79, 525–534. [Google Scholar] [CrossRef]
- Ritchey, T.W.; Seeley, H.W. Cytochromes in streptococcus faecalis var. Zymogenes grown in a haematin-containing medium. J. Gen. Microbiol. 1974, 85, 220–228. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van Tyne, D.; Martin, M.J.; Gilmore, M.S. Structure, Function, and Biology of the Enterococcus faecalis Cytolysin. Toxins 2013, 5, 895-911. https://doi.org/10.3390/toxins5050895
Van Tyne D, Martin MJ, Gilmore MS. Structure, Function, and Biology of the Enterococcus faecalis Cytolysin. Toxins. 2013; 5(5):895-911. https://doi.org/10.3390/toxins5050895
Chicago/Turabian StyleVan Tyne, Daria, Melissa J. Martin, and Michael S. Gilmore. 2013. "Structure, Function, and Biology of the Enterococcus faecalis Cytolysin" Toxins 5, no. 5: 895-911. https://doi.org/10.3390/toxins5050895




