Analysis of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Inoculated with Fusarium Graminearum
Abstract
:1. Introduction
2. Results and Discussions
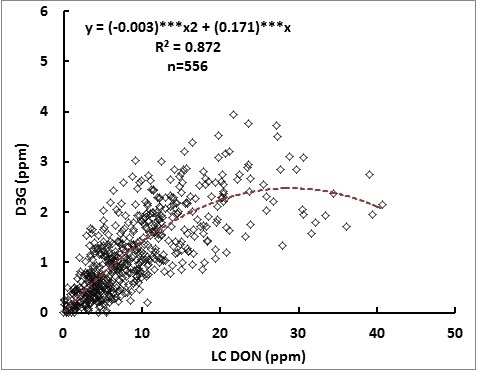
2.1. Relationship between DON and D3G

2.2. Effect of the Line, Location and Their Interactions on DON and D3G Content
| Year | Location | Range | DON a | D3G a |
|---|---|---|---|---|
| 2008 | Crookston | Min (n = 22) | 0.1 | 0.3 |
| Max (n = 22) | 24.2 | 1.8 | ||
| Average (n = 22) | 5.7 | 1.1 | ||
| St. Paul | Min (n = 22) | 0.7 | 0.1 | |
| Max (n = 22) | 39.5 | 1.9 | ||
| Average (n = 22) | 11.1 | 0.9 | ||
| MN | Min (n = 44) | 0.1 | 0.1 | |
| Max (n = 44) | 39.5 | 1.9 | ||
| Average (n = 44) | 8.4 | 1.0 | ||
| 2009 | Crookston | Min (n = 35) | 0.0 | 0.0 |
| Max (n = 35) | 25.7 | 3.8 | ||
| Average (n = 35) | 11.9 | 2.1 | ||
| St. Paul | Min (n = 35) | 0.2 | 0.0 | |
| Max (n = 35) | 21.0 | 1.5 | ||
| Average (n = 35) | 4.8 | 0.5 | ||
| MN | Min (n = 70) | 0.0 | 0.0 | |
| Max (n = 70) | 25.7 | 3.8 | ||
| Average (n = 70) | 8.3 | 1.3 | ||
| 2010 | Crookston | Min (n = 88) | 1.7 | 0.4 |
| Max (n = 88) | 20.2 | 2.6 | ||
| Average (n = 88) | 7.9 | 1.3 | ||
| St. Paul | Min (n = 90) | 0.2 | 0.0 | |
| Max (n = 90) | 11.5 | 1.5 | ||
| Average (n = 90) | 4.2 | 0.5 | ||
| MN | Min (n = 178) | 0.2 | 0.0 | |
| Max (n = 178) | 20.2 | 2.6 | ||
| Average (n = 178) | 6.1 | 0.9 |
| Year | Traits | Source | DF | Sum of squares | Mean square | F Value | Pr > F |
|---|---|---|---|---|---|---|---|
| 2008 | DON | Line | 21 | 3369.6 | 160.5 | 2.9 | 0.0095 |
| Loc | 1 | 418.7 | 418.7 | 7.5 | 0.0122 | ||
| Line × Loc | 21 | 1169.0 | 55.7 | 3.5 | 0.015 | ||
| Error | 12 | 191.7 | 16.0 | ||||
| D3G | Line | 21 | 12.7 | 0.60 | 3.8 | 0.0016 | |
| Loc | 1 | 0.9 | 0.88 | 5.6 | 0.0275 | ||
| Line × Loc | 21 | 3.3 | 0.16 | 4.7 | 0.004 | ||
| Error | 12 | 0.4 | 0.03 | ||||
| 2009 | DON | Line | 34 | 5314.4 | 156.3 | 5.3 | <0.001 |
| Loc | 1 | 1013.4 | 1013.4 | 34.3 | <0.001 | ||
| Line × Loc | 34 | 1004.5 | 29.5 | 1.3 | 0.177 | ||
| Error | 72 | 1639.7 | 22.8 | ||||
| D3G | Line | 34 | 27.9 | 0.82 | 2.5 | 0.0048 | |
| Loc | 1 | 49.3 | 49.28 | 149.0 | <0.0001 | ||
| Line × Loc | 34 | 11.2 | 0.33 | 2.6 | 0.000 | ||
| Error | 72 | 9.0 | 0.13 | ||||
| 2010 | DON | Line | 89 | 3479.0 | 39.1 | 8.0 | <0.0001 |
| Loc | 1 | 650.1 | 650.1 | 132.5 | <0.0001 | ||
| Line × Loc | 87 | 426.8 | 4.9 | 1.0 | 0.497 | ||
| Error | 88 | 419.0 | 4.8 | ||||
| D3G | Line | 89 | 48.1 | 0.54 | 4.0 | <0.0001 | |
| Loc | 1 | 33.1 | 33.09 | 247.4 | <0.0001 | ||
| Line × Loc | 87 | 11.6 | 0.13 | 1.0 | 0.497 | ||
| Error | 88 | 11.8 | 0.13 |
2.3. Correlation of DON and D3G Content between Locations
| Year | Crk DON | Stp DON | Crk D3G | Stp D3G |
|---|---|---|---|---|
| 2008 | Pearson Correlation | |||
| - | 0.59 ** | 0.56 ** | 0.56 ** | |
| 0.51 * | - | 0.47 * | 0.90 *** | |
| 0.68 *** | 0.46 * | - | 0.59 ** | |
| 0.58 ** | 0.87 *** | 0.51 * | - | |
| Spearman correlation | ||||
| 2009 | Pearson Correlation | |||
| - | 0.52 ** | 0.66 *** | 0.50 ** | |
| 0.45 ** | - | 0.24 NS | 0.75 *** | |
| 0.56 *** | 0.44 ** | - | 0.41 * | |
| 0.49 ** | 0.69 *** | 0.56 *** | - | |
| Spearman correlation | ||||
| 2010 | Pearson Correlation | |||
| - | 0.63 *** | 0.69 *** | 0.41 *** | |
| 0.57 *** | - | 0.51 *** | 0.67 *** | |
| 0.55 *** | 0.45 *** | - | 0.48 *** | |
| 0.34 ** | 0.61 *** | 0.44 *** | - | |
| Spearman correlation | ||||
3. Experimental Section
3.1. Standards and Chemicals
3.2. Samples
3.3. Sample Preparation
3.4. LC-MS Instrumentation and Methodology
3.5. Statistics Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar]
- European Food Safety Authority. European Food Safety Authority Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379–3435. [Google Scholar]
- Wegulo, S. Factors influencing deoxinivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of different trichothecenes and deoxynivalenol-3-β-d-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin. Res. 2012, 28, 181–190. [Google Scholar] [CrossRef]
- Lemmens, M.; Koutnik, A.; Steiner, B.; Buerstmayr, H.; Berthiller, F.; Schuhmacher, R.; Maier, F.; Schäfer, W. Investigations on the ability of Fhb1 to protect wheat against nivalenol and deoxynivalenol. Cereal Res. Commun. 2008, 36, 429–435. [Google Scholar]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. Royal Soc. Interface 2011, 8, 117–126. [Google Scholar]
- Maul, R.; Müller, C.; Rieß, S.; Koch, M.; Methner, F.J.; Irene, N. Germination induces the glucosylation of the Fusarium mycotoxin deoxynivalenol in various grains. Food Chem. 2012, 131, 274–279. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glossl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef]
- Shin, S.; Torres-Acosta, J.A.; Heinen, S.J.; McCormick, S.; Lemmens, M.; Paris, M.P.K.; Berthiller, F.; Adam, G.; Muehlbauer, G.J. Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. J. Exp. Botany 2012, 63, 4731–4740. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, Á.; Krska, R. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant-Mic. Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar]
- Malachova, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: The major mycotoxins found in cereal-based products on the Czech market. J. Agricul. Food Chem. 2011, 59, 12990–12997. [Google Scholar] [CrossRef]
- Sasanya, J.J.; Hall, C.; Wolf-Hall, C. Analysis of deoxynivalenol, masked deoxynivalenol, and Fusarium graminearum pigment in wheat samples, using liquid chromatographyuvmass spectrometry. J. Food Prot. 2008, 71, 1205–1213. [Google Scholar]
- Dall’Asta, C.; Dall’Erta, A.; Mantovani, P.; Massi, A.; Galaverna, G. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat. World Mycotoxin J. 2013, 6, 83–91. [Google Scholar] [CrossRef]
- Cowger, C.; Patton-Ozkurt, J.; Brown-Guedira, G.; Perugini, L. Post-anthesis moisture increased Fusarium head blight and deoxynivalenol levels in North Carolina winter wheat. Phytopathology 2009, 99, 320–327. [Google Scholar] [CrossRef]
- Tacke, B.K.; Casper, H.H. Determination of deoxynivalenol in wheat, barley, and malt by column cleanup and gas chromatography with electron capture detection. J. AOAC Intern. 1996, 79, 472–475. [Google Scholar]
- Simsek, S.; Burgess, K.; Whitney, K.L.; Gu, Y.; Qian, S.Y. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in wheat. Food Control 2012, 26, 287–292. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.-B.; Simsek, S. Analysis of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Inoculated with Fusarium Graminearum. Toxins 2013, 5, 2522-2532. https://doi.org/10.3390/toxins5122522
Ovando-Martínez M, Ozsisli B, Anderson J, Whitney K, Ohm J-B, Simsek S. Analysis of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Inoculated with Fusarium Graminearum. Toxins. 2013; 5(12):2522-2532. https://doi.org/10.3390/toxins5122522
Chicago/Turabian StyleOvando-Martínez, Maribel, Bahri Ozsisli, James Anderson, Kristin Whitney, Jae-Bom Ohm, and Senay Simsek. 2013. "Analysis of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Inoculated with Fusarium Graminearum" Toxins 5, no. 12: 2522-2532. https://doi.org/10.3390/toxins5122522