Glucan–Resveratrol–Vitamin C Combination Offers Protection against Toxic Agents
Abstract
:1. Introduction
2. Effects on Mercury

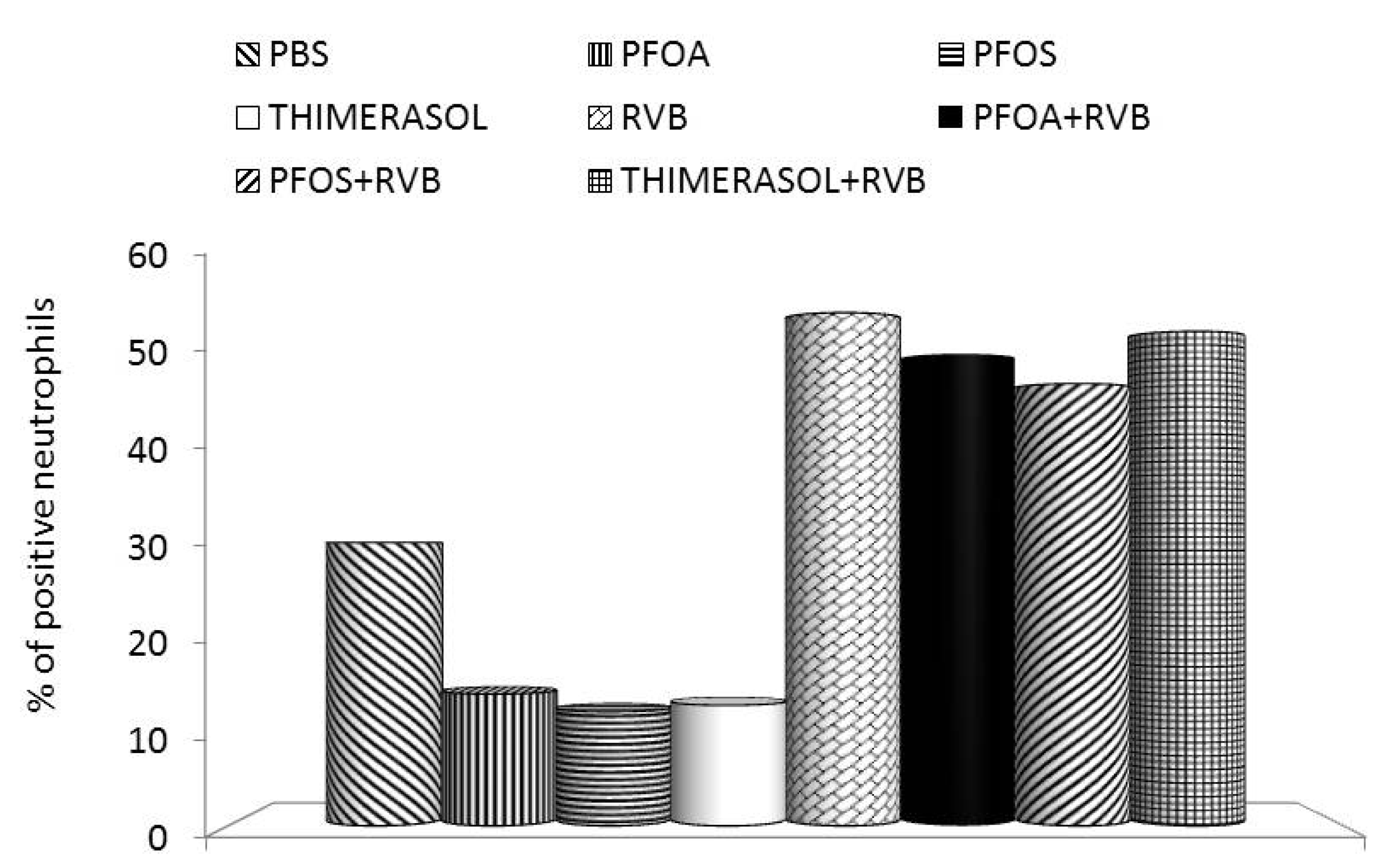
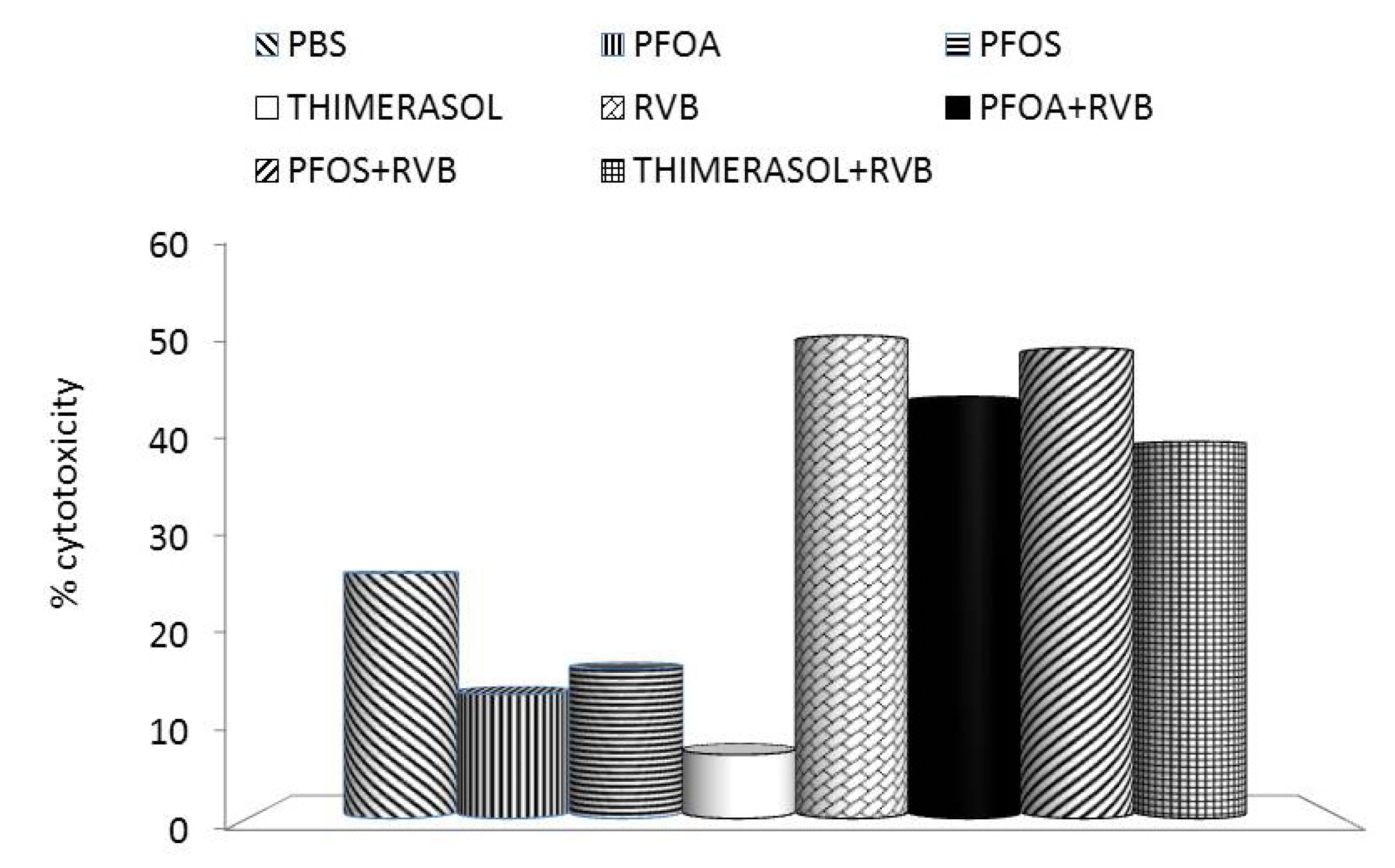
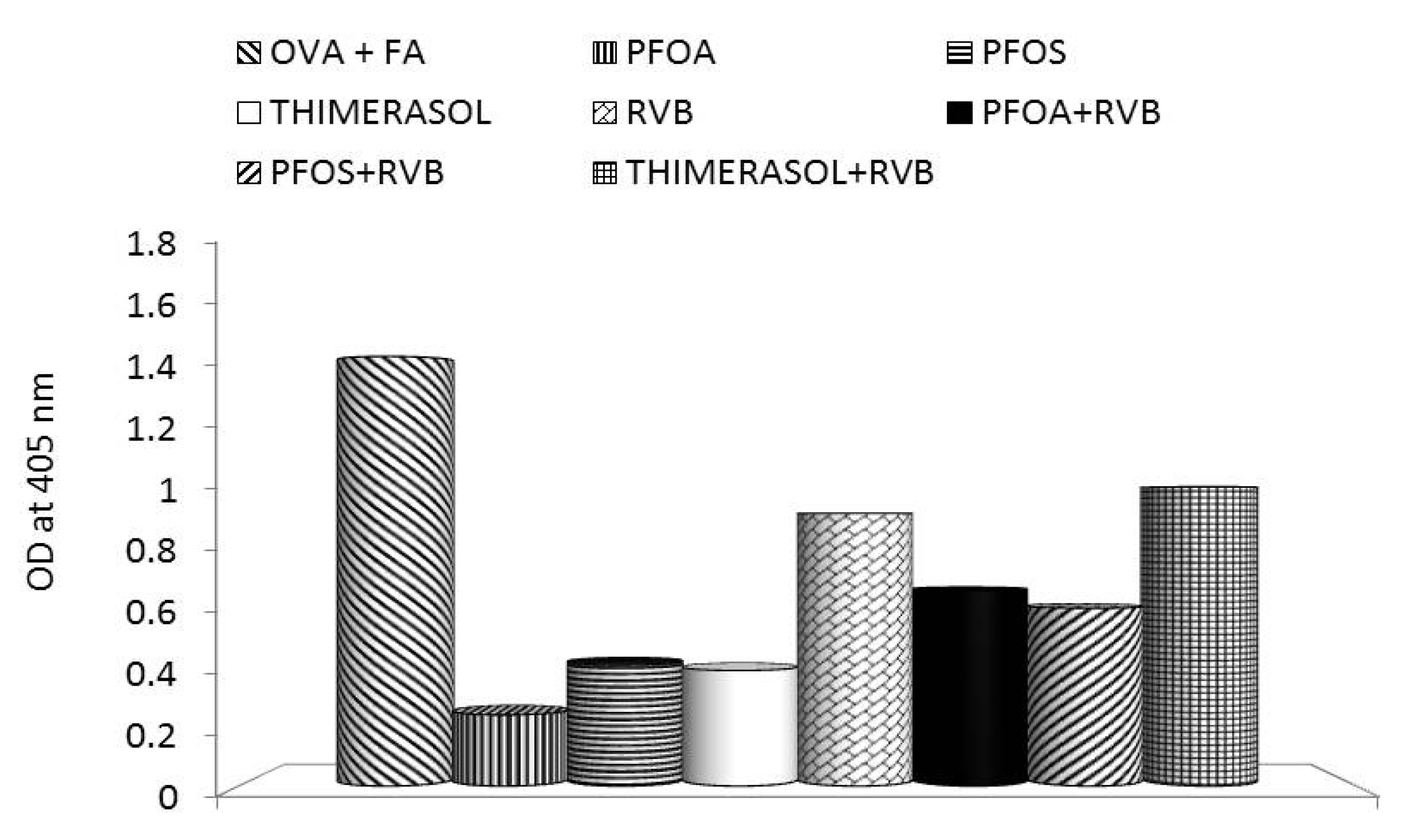
3. Effects of PFCs
4. Conclusions
Conflict of Interest
References
- Tryphonas, H. Approaches to detecting immunotoxic effects of environmental contaminants in humans. Environ. Health Perspect. 2001, 109, 877–884. [Google Scholar]
- Magos, L. Review of the toxicity of ethylmercury, including its presence as a preservative in biological and pharmaceutical products. J. Appl. Toxicol. 2001, 21, 1–5. [Google Scholar]
- Yole, M.; Wickstrom, M.; Blakley, B. Cell death and cytotoxic effects in YAC-1 lymphome cells following exposure to variosu forms of mercury. Toxicol. J. 2007, 231, 40–57. [Google Scholar] [CrossRef]
- Santarelli, L.; Bracii, M.; Mocchyeegiani, E. In vitro and in vivo effects of mercuric chloride on thymic endocrine activity, NK and NKT cell cytotoxicity, cytokine profiles (IL-2, INF-g, IL-6): Role of the nitric oxide-L-arginine pathway. Int. Immunopathol. 2006, 6, 376–389. [Google Scholar]
- Descotes, J. Immunotoxicity of Drugs and Chemicals: An Experimental and Clinical Approach; Elsevier: Amsterdam, The netherlands, 2004. [Google Scholar]
- Fillipov, N.M.; Pinchuk, L.M.; Boyd, B.L.; Crittenden, P.L. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci. 2005, 86, 324–332. [Google Scholar]
- Khan, N.M.; Poduval, T.B. Immunomodulatory and immunotoxic effects of bilirubin: Molecular mechanisms. J. Leukoc. Biol. 2011, 90, 997–1015. [Google Scholar] [CrossRef]
- Vetvicka, V.; Fornusek, L.; Sima, P.; Bilej, M.; Táborsky, L.; Rihova, B.; Simeckova, J.; Miler, I. Effects of bilirubin on murine peritoneal and spleen cells. Acta Pathol. Microbiol. Immunol. Scand. 1988, 96, 671–675. [Google Scholar]
- Dass, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar]
- Benacerraf, B.; Sebestyen, M.M. Effect of bacterial endotoxins on the reticuloendothelial system. Fed. Proc. 1957, 16, 860–867. [Google Scholar]
- Vetvicka, V.; Novak, M. Biological Action of β-glucan. In Biology and Chemistry of Beta Glucan; Vetvicka, V., Novak, M., Eds.; Bentham Science Publishers: Oak Park, IL, USA, 2011; pp. 10–18. [Google Scholar]
- Novak, M.; Vetvicka, V. Glucans as biological response modifiers. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 67–75. [Google Scholar]
- Ditteova, G.; Velebny, S.; Hrckova, G. Modulation of liver fibrosis and pathophysiological changes in mice infected with Mesocestoides corti (M. vogae) after administration of glucan and liposomized glucan in combination with vitamin C. J. Helminthol. 2003, 77, 219–226. [Google Scholar]
- Verlhac, V.; Gabaudan, J.; Obach, A.; Schuep, W.; Hole, R. Influence of dietary glucan and vitamin C on non-specifc and specific immune responses of rainbow trout (Onchorhynchus mykiss). Aquaculture 1996, 143, 123–133. [Google Scholar] [CrossRef]
- Vetvicka, V.; Baigorri, R.; Zamarreno, A.M.; Garcia-Minam, J.M.; Yvin, J.C. Glucan and humic acids: Synergistic effects on the immune system. J. Med. Food 2010, 13, 863–869. [Google Scholar] [CrossRef]
- Vetvicka, V.; Volny, T.; Saraswat-Ohri, S.; Vashishta, A.; Vancikova, Z.; Vetvickova, J. Glucan and resveratrol complex—Possible synergistic effects on immune system. Biomed. Pap. Med. Fac. Univ. Palacky 2007, 151, 41–46. [Google Scholar]
- Vetvicka, V.; Vetvickova, J. Combination of glucan, resveratrol and vitamin C demonstrates strong anti-tumor potential. Anticancer Res. 2012, 32, 81–88. [Google Scholar]
- Vetvicka, V.; Vetvickova, J. Effects of glucan on immunosuppressive action of mercury. J. Med. Food 2009, 12, 1098–1104. [Google Scholar] [CrossRef]
- Vetvicka, V. β-Glucans as immunomodulators. JANA 2001, 3, 31–34. [Google Scholar]
- Vetvicka, V.; Dvorak, B.; Vetvickova, J.; Richter, J.; Krizan, J.; Sima, P.; Yvin, J.C. Orally administered marine (1→3)-β-D-glucan Phycarine stimulates both humoral and cellular immunity. Int. J. Biol. Macromol. 2007, 40, 291–298. [Google Scholar] [CrossRef]
- Novak, M.; Vetvicka, V. Development of Views on β-Glucan Composition and Structure. In Biology and Chemistry of Beta Glucan; Vetvicka, V., Novak, M., Eds.; Bentham Science Publishers: Oak Park, IL, USA, 2011; pp. 1–9. [Google Scholar]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvickova, J. Immunological effects of yeast- and mushroom-derived beta-glucans. J. Med. Food 2008, 11, 615–622. [Google Scholar] [CrossRef]
- Sarafian, T.A. Methylmercury-induced generatrion of free radicals: biological implications. Met. Ions Biol. Syst. 1996, 36, 415–444. [Google Scholar]
- Vetvicka, V.; Vetvickova, J. β-1,3-Glucan: Silver bullet or hot air? Open Glycosci. 2010, 3, 1–6. [Google Scholar]
- Quig, D. Cysteine metabolism and metal toxicity. Altern. Med. Rev. 1998, 3, 262–270. [Google Scholar]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 66–94. [Google Scholar]
- Dong, G.H.; Zhang, Y.H.; Zheng, L.; Liu, W.; Jin, Y.H.; He, Q.C. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch. Toxicol. 2009, 83, 805–815. [Google Scholar] [CrossRef]
- Qazi, M.R.; Bogdanska, J.; Butenhoff, J.L.; Nelson, B.D.; DePierre, J.W.; Bedi-Valugerdi, M. High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicol. J. 2009, 262, 207–214. [Google Scholar] [CrossRef]
- Corsini, E.; Sangiovanni, E.; Avogadro, A.; Galbiati, V.; Viviani, B.; Marinovich, M.; Galli, C.L.; Dell’Agli, M.; Germolec, D.R. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol. 2012, 258, 248–255. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar]
- Grandjean, P.; Andersen, E.W.; Budtz-Jorgensen, E.; Nielsen, F.; Molbak, K.; Weihe, P.; Heilmann, C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. J. Am. Med. Assoc. 2012, 307, 391–397. [Google Scholar]
- Calafat, A.N.; Wong, L.Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the national health and nutrition examination survey (NHANES) 2003–2004 and comparison with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. An evaluation of the immunological activities of commercially available β1,3-glucans. JANA 2007, 10, 25–31. [Google Scholar]
- Eicher, S.D.; McKee, C.A.; Carroll, J.A.; Pajor, E.A. Supplemental vitamin C and yeast cell wall beta-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning. J. Anim. Sci. 2006, 84, 2352–2360. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Comparison of immunological properties of various bioactive combinations. Biomed. Pap. Med. Fac. Univ. Palacky 2012, in press.. [Google Scholar]
- Bowers, G.J.; Patchen, M.L.; MacVittie, T.J.; Hirsch, E.F.; Fink, M.P. A comparative evaluation of particulate and soluble glucan in an endotoxin model. Int. J. Immunopharmacol. 1986, 8, 313–321. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vetvicka, V.; Vetvickova, J. Glucan–Resveratrol–Vitamin C Combination Offers Protection against Toxic Agents. Toxins 2012, 4, 1301-1308. https://doi.org/10.3390/toxins4111301
Vetvicka V, Vetvickova J. Glucan–Resveratrol–Vitamin C Combination Offers Protection against Toxic Agents. Toxins. 2012; 4(11):1301-1308. https://doi.org/10.3390/toxins4111301
Chicago/Turabian StyleVetvicka, Vaclav, and Jana Vetvickova. 2012. "Glucan–Resveratrol–Vitamin C Combination Offers Protection against Toxic Agents" Toxins 4, no. 11: 1301-1308. https://doi.org/10.3390/toxins4111301




