Uncoupling of T Cell Receptor Zeta Chain Function during the Induction of Anergy by the Superantigen, Staphylococcal Enterotoxin A
Abstract
:1. Introduction
2. Results and Discussion
2.1. The role of APCs in the SEA-induced proliferation of the A.E7 T cell clone
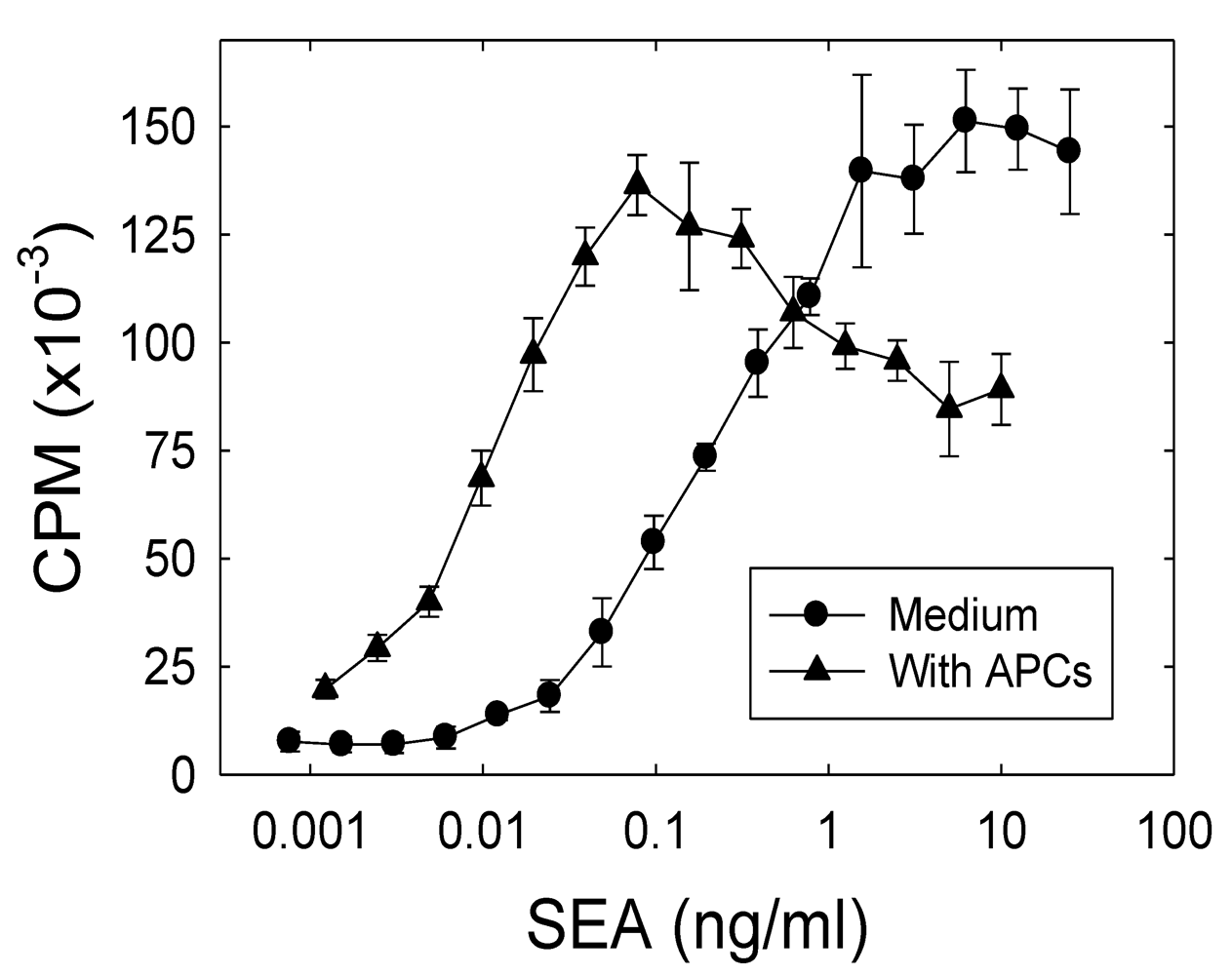

2.2. SEA-induced T cell anergy

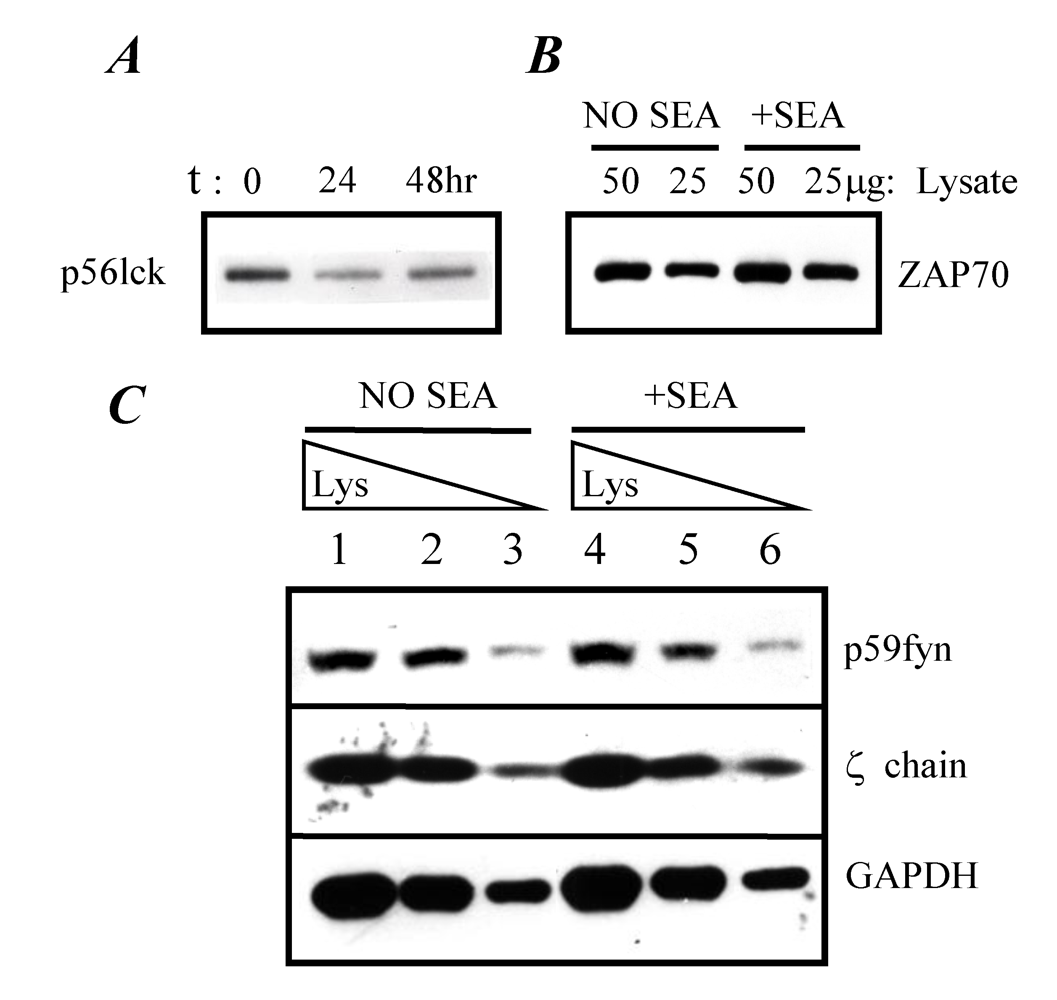
2.3. Expression of TCR-proximal signaling components in anergized A.E7 cells
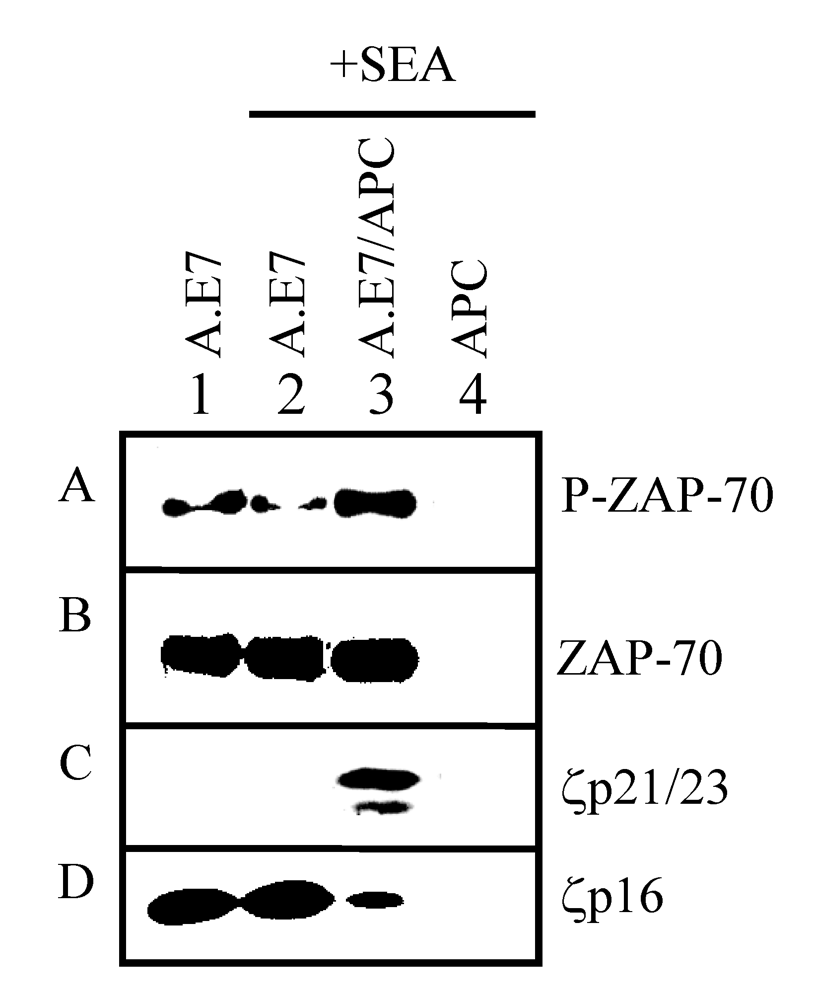
2.4. TCR-proximal signaling components during the induction phase of anergy
3. Experimental Section
3.1. Reagents
3.2. T cell activation
3.3. Anergy induction
3.4. TCR signaling analysis
4. Conclusions
Acknowledgements
References
- Marrack, P.; Kappler, J. The staphylococcal enterotoxins and their relatives. Science 1990, 248, 705–711. [Google Scholar]
- Schlievert, P.M. Role of superantigens in human disease. J. Infect. Dis. 1993, 167, 997–1002. [Google Scholar]
- Fraser, J.D.; Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar]
- Qian, D.; Griswold-Prenner, I.; Rosner, M.R.; Fitch, F.W. Multiple components of the T cell antigen receptor complex become tyrosine-phosphorylated upon activation. J. Biol. Chem. 1993, 268, 4488–4493. [Google Scholar]
- Marchildon, G.A.; Casnellie, J.E.; Walsh, K.A.; Krebs, E.G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc. Natl. Acad. Sci. USA 1984, 81, 7679–7682. [Google Scholar]
- Rudd, C.E.; Trevillyan, J.M.; Dasgupta, J.D.; Wong, L.L.; Schlossman, S.F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 5190–5194. [Google Scholar]
- Abraham, N.; Veillette, A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol. Cell Biol. 1990, 10, 5197–5206. [Google Scholar]
- Veillette, A.; Bookman, M.A.; Horak, E.M.; Bolen, J.B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 1988, 55, 301–308. [Google Scholar]
- Danielian, S.; Fagard, R.; Alcover, A.; Acuto, O.; Fischer, S. The lymphocyte-specific protein tyrosine kinase p56lck is hyperphosphorylated on serine and tyrosine residues within minutes after activation via T cell receptor or CD2. Eur. J. Immunol. 1989, 19, 2183–2189. [Google Scholar]
- Tsygankov, A.Y.; Broker, B.M.; Fargnoli, J.; Ledbetter, J.A.; Bolen, J.B. Activation of tyrosine kinase p60fyn following T cell antigen receptor cross-linking. J. Biol. Chem. 1992, 267, 18259–18262. [Google Scholar]
- Smith, J.A.; Tso, J.Y.; Clark, M.R.; Cole, M.S.; Bluestone, J.A. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J. Exp. Med. 1997, 185, 1413–1422. [Google Scholar]
- Jenkins, M.K.; Schwartz, R.H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987, 165, 302–319. [Google Scholar]
- DeSilva, D.R.; Urdahl, K.B.; Jenkins, M.K. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J. Immunol. 1991, 147, 3261–3267. [Google Scholar]
- Boussiotis, V.A.; Gribben, J.G.; Freeman, G.J.; Nadler, L.M. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr. Opin. Immunol. 1994, 6, 797–807. [Google Scholar]
- Smith, J.A.; Bluestone, J.A. T cell inactivation and cytokine deviation promoted by anti-CD3 mAbs. Curr. Opin. Immunol. 1997, 9, 648–654. [Google Scholar]
- Sloan-Lancaster, J.; Evavold, B.D.; Allen, P.M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature 1993, 363, 156–159. [Google Scholar]
- Sloan-Lancaster, J.; Shaw, A.S.; Rothbard, J.B.; Allen, P.M. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell 1994, 79, 913–922. [Google Scholar]
- Taub, D.D.; Rogers, T.J. Direct activation of murine T cells by staphylococcal enterotoxins. Cell Immunol. 1992, 140, 267–281. [Google Scholar]
- Cornwell, W.D.; Rogers, T.J. Role of interleukin-2 in superantigen-induced T-cell anergy. Immunology 1999, 96, 193–201. [Google Scholar]
- O'Hehir, R.E.; Lamb, J.R. Induction of specific clonal anergy in human T lymphocytes by Staphylococcus aureus enterotoxins. Proc. Natl. Acad. Sci. USA 1990, 87, 8884–8888. [Google Scholar] [CrossRef]
- Rammensee, H.G.; Kroschewski, R.; Frangoulis, B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature 1989, 339, 541–544. [Google Scholar]
- Kawabe, Y.; Ochi, A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J. Exp. Med. 1990, 172, 1065–1070. [Google Scholar]
- Rellahan, B.L.; Jones, L.A.; Kruisbeek, A.M.; Fry, A.M.; Matis, L.A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J. Exp. Med. 1990, 172, 1091–1100. [Google Scholar]
- MacDonald, H.R.; Baschieri, S.; Lees, R.K. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur. J. Immunol. 1991, 21, 1963–1966. [Google Scholar]
- Miethke, T.; Wahl, C.; Heeg, K.; Wagner, H. Acquired resistance to superantigen-induced T cell shock. V beta selective T cell unresponsiveness unfolds directly from a transient state of hyperreactivity. J. Immunol. 1993, 150, 3776–3784. [Google Scholar] [PubMed]
- Hewitt, C.R.; Lamb, J.R.; Hayball, J.; Hill, M.; Owen, M.J.; O'Hehir, R.E. Major histocompatibility complex independent clonal T cell anergy by direct interaction of Staphylococcus aureus enterotoxin B with the T cell antigen receptor. J. Exp. Med. 1992, 175, 1493–1499. [Google Scholar]
- Kotb, M.; Watanabe-Ohnishi, R.; Aelion, J.; Tanaka, T.; Geller, A.M.; Ohnishi, H. Preservation of the specificity of superantigen to T cell receptor V beta elements in the absence of MHC class II molecules. Cell Immunol. 1993, 152, 348–357. [Google Scholar]
- Powell, J.D.; Lerner, C.G.; Schwartz, R.H. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J. Immunol. 1999, 162, 2775–2784. [Google Scholar]
- Quill, H.; Riley, M.P.; Cho, E.A.; Casnellie, J.E.; Reed, J.C.; Torigoe, T. Anergic Th1 cells express altered levels of the protein tyrosine kinases p56lck and p59fyn. J. Immunol. 1992, 149, 2887–2893. [Google Scholar]
- Gajewski, T.F.; Qian, D.; Fields, P.; Fitch, F.W. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc. Natl. Acad. Sci. USA 1994, 91, 38–42. [Google Scholar]
- Gajewski, T.F.; Fields, P.; Fitch, F.W. Induction of the increased Fyn kinase activity in anergic T helper type 1 clones requires calcium and protein synthesis and is sensitive to cyclosporin A. Eur. J. Immunol. 1995, 25, 1836–1842. [Google Scholar]
- Madrenas, J.; Schwartz, R.H.; Germain, R.N. Interleukin 2 production, not the pattern of early T-cell antigen receptor-dependent tyrosine phosphorylation, controls anergy induction by both agonists and partial agonists. Proc. Natl. Acad. Sci. USA 1996, 93, 9736–9741. [Google Scholar]
- Cauley, L.S.; Cauley, K.A.; Shub, F.; Huston, G.; Swain, S.L. Transferable anergy: superantigen treatment induces CD4+ T cell tolerance that is reversible and requires CD4-CD8- cells and interferon gamma. J. Exp. Med. 1997, 186, 71–81. [Google Scholar]
- Belfrage, H.; Dohlsten, M.; Hedlund, G.; Kalland, T. Prevention of superantigen-induced tolerance in vivo by interleukin-2 treatment. Cancer Immunol. 1997, 44, 77–82. [Google Scholar]
- Aoki, Y.; Yoshikai, Y. Further clonal expansion of T cells upon rechallenge of superantigen staphylococcal enterotoxin A. Micro. Immunol. 1997, 41, 337–343. [Google Scholar]
- Sundstedt, A.; Sigvardsson, M.; Leanderson, T.; Hedlund, G.; Kalland, T. In vivo anergized CD4+ T cells express perturbed AP-1 and NF-kappa B transcription factors. Pharm. Onco. Immunol. 1996, 93, 979–984. [Google Scholar]
- Migita, K.; Eguchi, K.; Kawabe, Y.; Tsukada, T.; Ichinose, Y.; Nagataki, S. Defective TCR-mediated signaling in anergic T cells. J. Immunol. 1995, 155, 5083–5087. [Google Scholar]
- Watson, A.R.O.; Lee, W.T. Defective T cell receptor-mediated signal transduction in memory CD4 T lymphocytes exposed to superantigen or anti-T cell receptor antibodies. Cell. Immunol. 2006, 242, 80–90. [Google Scholar]
- Fujimaki, W.; Iwashima, M.; Yagi, J.; Zhang, H.; Yagi, H.; Seo, K.; Imai, Y.; Imanishi, K.; Uchiyama, T. Functional uncoupling of T cell receptor engagement and lck activation in anergic human thymic CD4 T cells. J. Biol. Chem. 2001, 276, 17455–17460. [Google Scholar]
- Subramanyam, M.; Mohan, N.; Mottershead, D.; Beutner, U.; McLellan, B.; Kraus, E.; Huber, B.T. Mls-1 superantigen: molecular characterization and functional analysis. Immunol. Rev. 1993, 131, 117–130. [Google Scholar]
- Sacchi, A.; Tempestilli, M.; Turchi, F.; Agrati, C.; Casetti, R.; Cimini, E.; Gioia, C.; Martini, F. CD3zeta down-modulation may explain Vgamma9Vdelta2 T lymphocyte anergy in HIV-infected patients. J. Infect. Dis. 2009, 199, 432–436. [Google Scholar]
- Simone, R.; Piatti, G.; Saverino, D. The inhibitory co-receptors: a way to save from anergy the HIV-specific T cells. Curr. HIV Res. 2009, 7, 266–272. [Google Scholar]
- Podojil, J.R.; Turley, D.M.; Miller, S.D. Therapeutic blockade of T-cell antigen receptor signal transduction and costimulation in autoimmune disease. Adv. Exp. Med. Biol. 2008, 640, 234–251. [Google Scholar]
- Podojil, J.R.; Miller, S.D. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol. Rev. 2009, 229, 337–355. [Google Scholar]
- Matis, L.A.; Longo, D.L.; Hedrick, S.M.; Hannum, C.; Margoliash, E.; Schwartz, R.H. Clonal analysis of the major histocompatibility complex restriction and the fine specificity of antigen recognition in the T cell proliferative response to cytochrome C. J. Immunol. 1983, 130, 1527–1535. [Google Scholar]
- Madrenas, J.; Wange, R.L.; Wang, J.L.; Isakov, N.; Samelson, L.E.; Germain, R.N. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science 1995, 267, 515–518. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cornwell, W.D.; Rogers, T.J. Uncoupling of T Cell Receptor Zeta Chain Function during the Induction of Anergy by the Superantigen, Staphylococcal Enterotoxin A. Toxins 2010, 2, 1704-1717. https://doi.org/10.3390/toxins2071704
Cornwell WD, Rogers TJ. Uncoupling of T Cell Receptor Zeta Chain Function during the Induction of Anergy by the Superantigen, Staphylococcal Enterotoxin A. Toxins. 2010; 2(7):1704-1717. https://doi.org/10.3390/toxins2071704
Chicago/Turabian StyleCornwell, William D., and Thomas J. Rogers. 2010. "Uncoupling of T Cell Receptor Zeta Chain Function during the Induction of Anergy by the Superantigen, Staphylococcal Enterotoxin A" Toxins 2, no. 7: 1704-1717. https://doi.org/10.3390/toxins2071704



