Prophylactic Effects of Bee Venom Phospholipase A2 in Lipopolysaccharide-Induced Pregnancy Loss
Abstract
:1. Introduction
2. Results
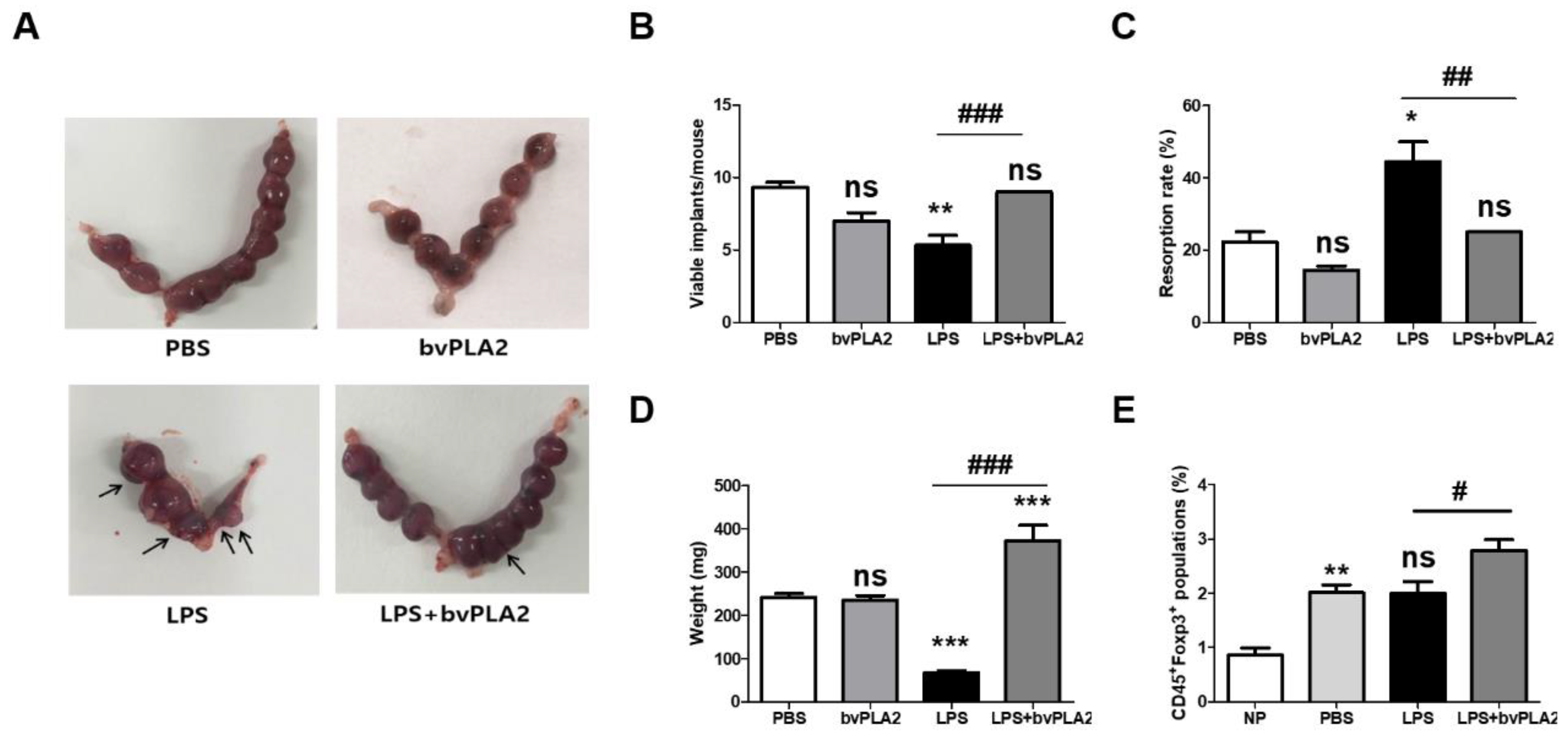
2.1. Effect of bvPLA2 Pretreatment on Fetal Loss in LPS-Induced Abortion Mice
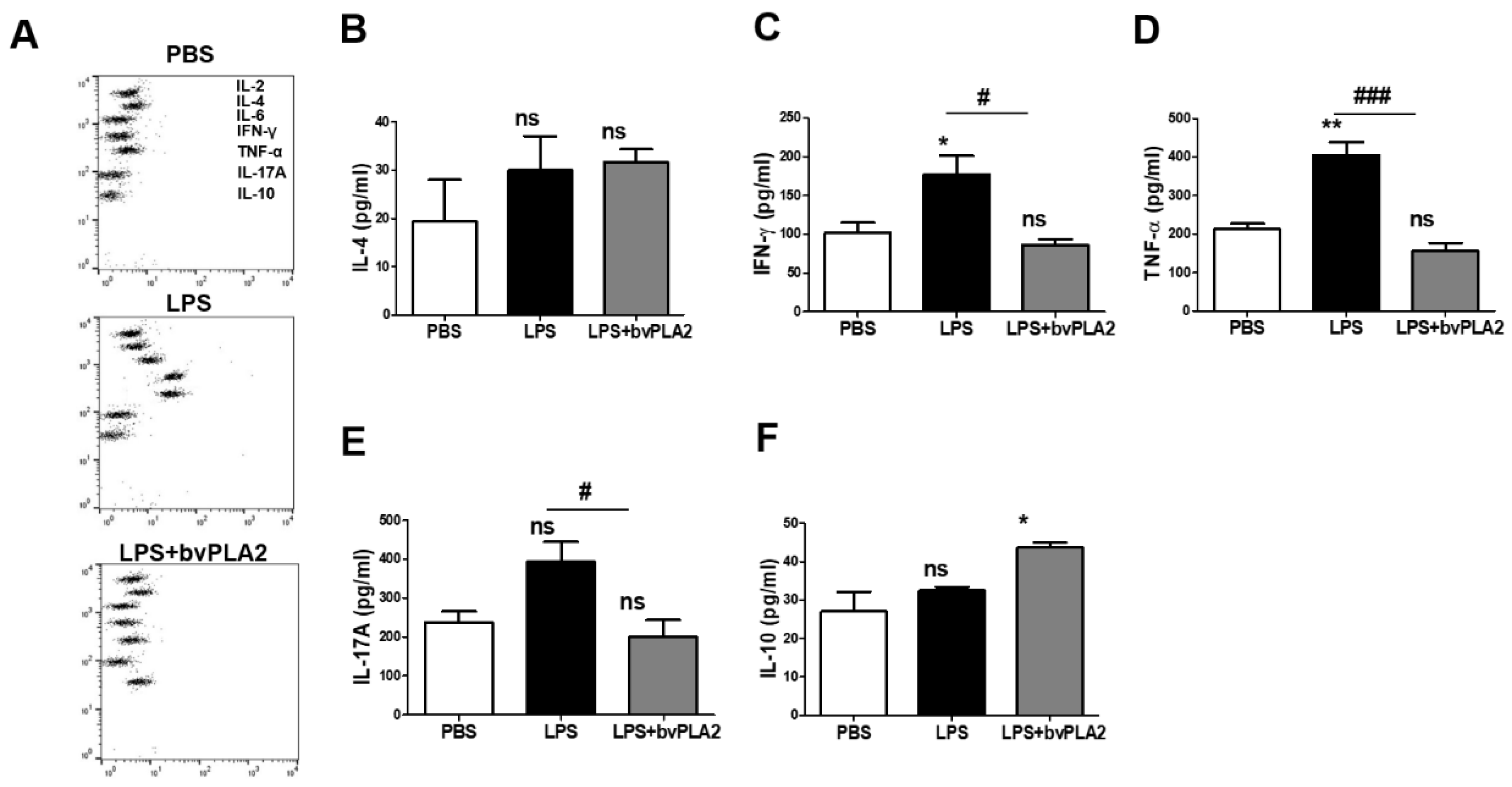
2.2. Effect of bvPLA2 on the Inflammatory Cytokine Levels in LPS-Induced Abortion Mice
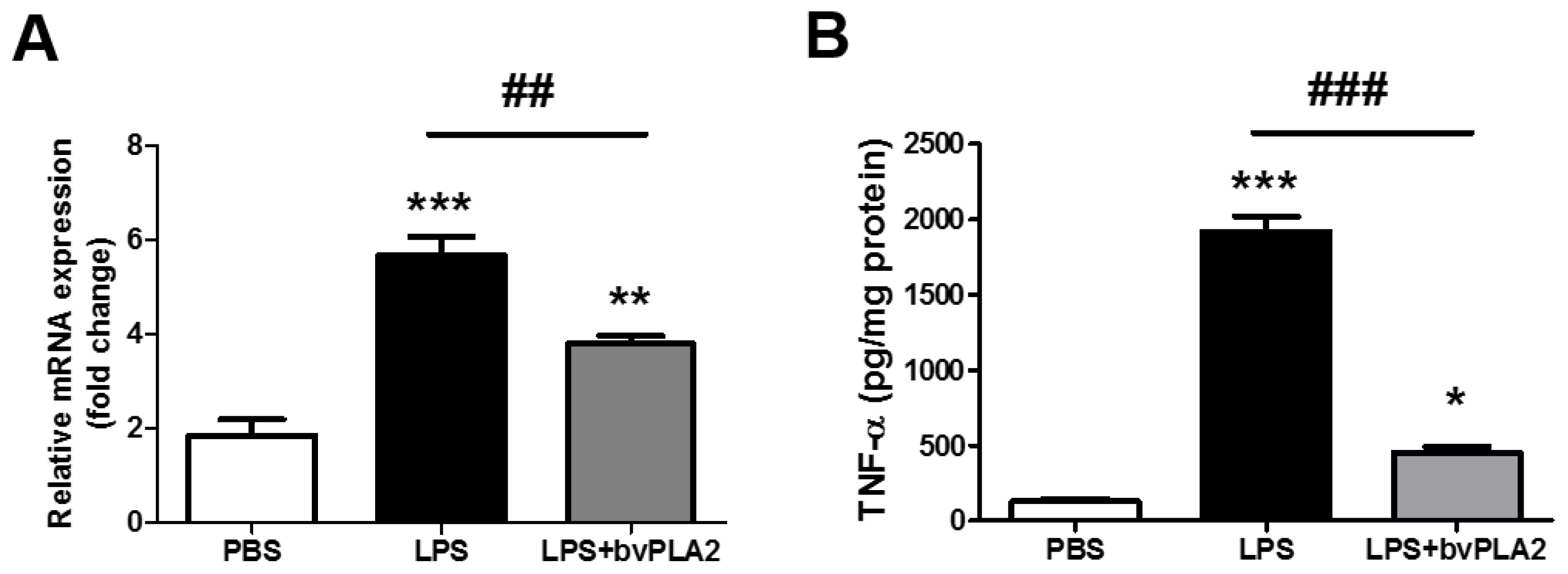
2.3. bvPLA2 Pretreatment Inhibits LPS-Induced TNF-α Production
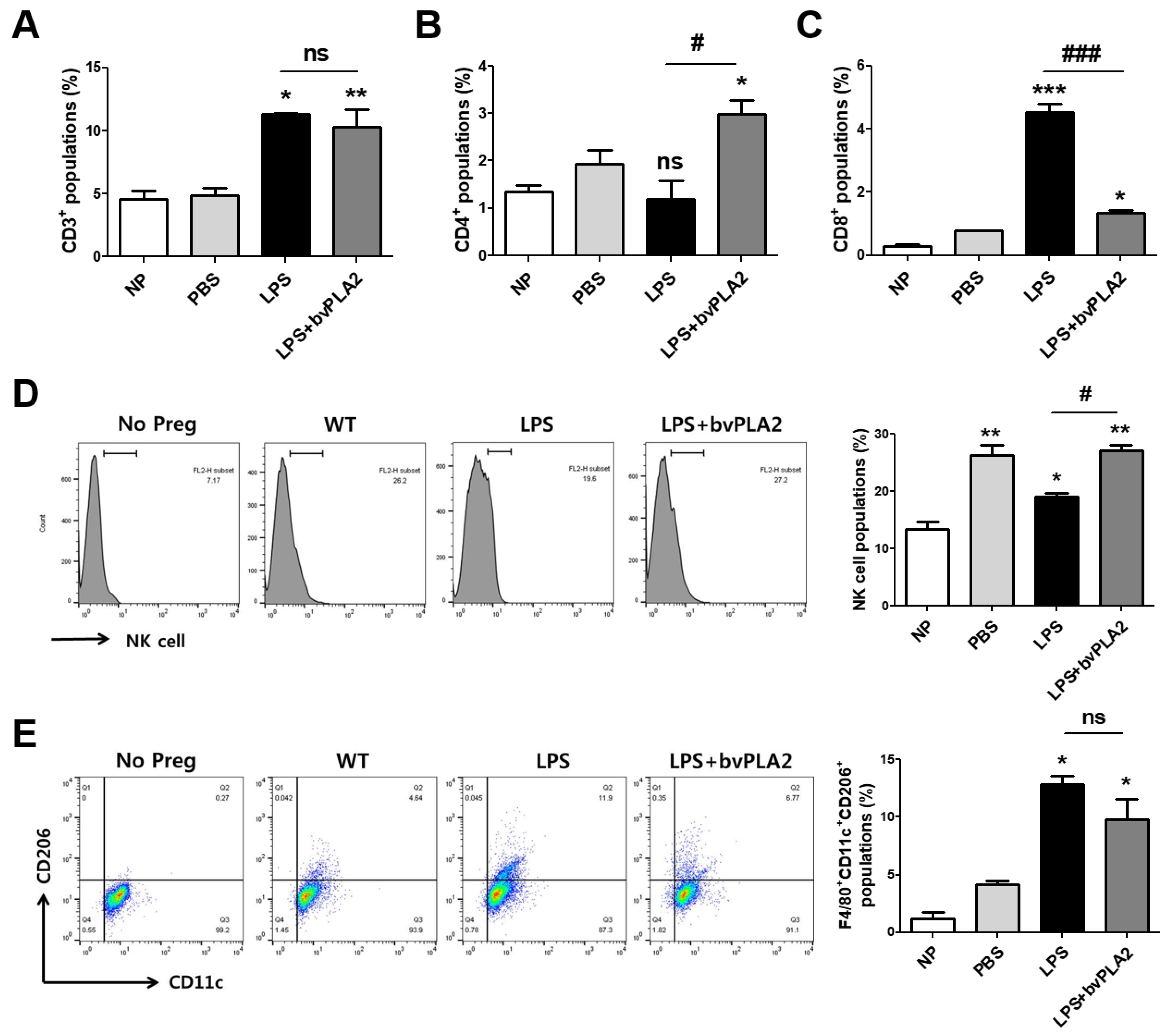
2.4. Effects of bvPLA2 on Various Immune Cell Types in Mice Prone to LPS-Induced Spontaneous Abortion
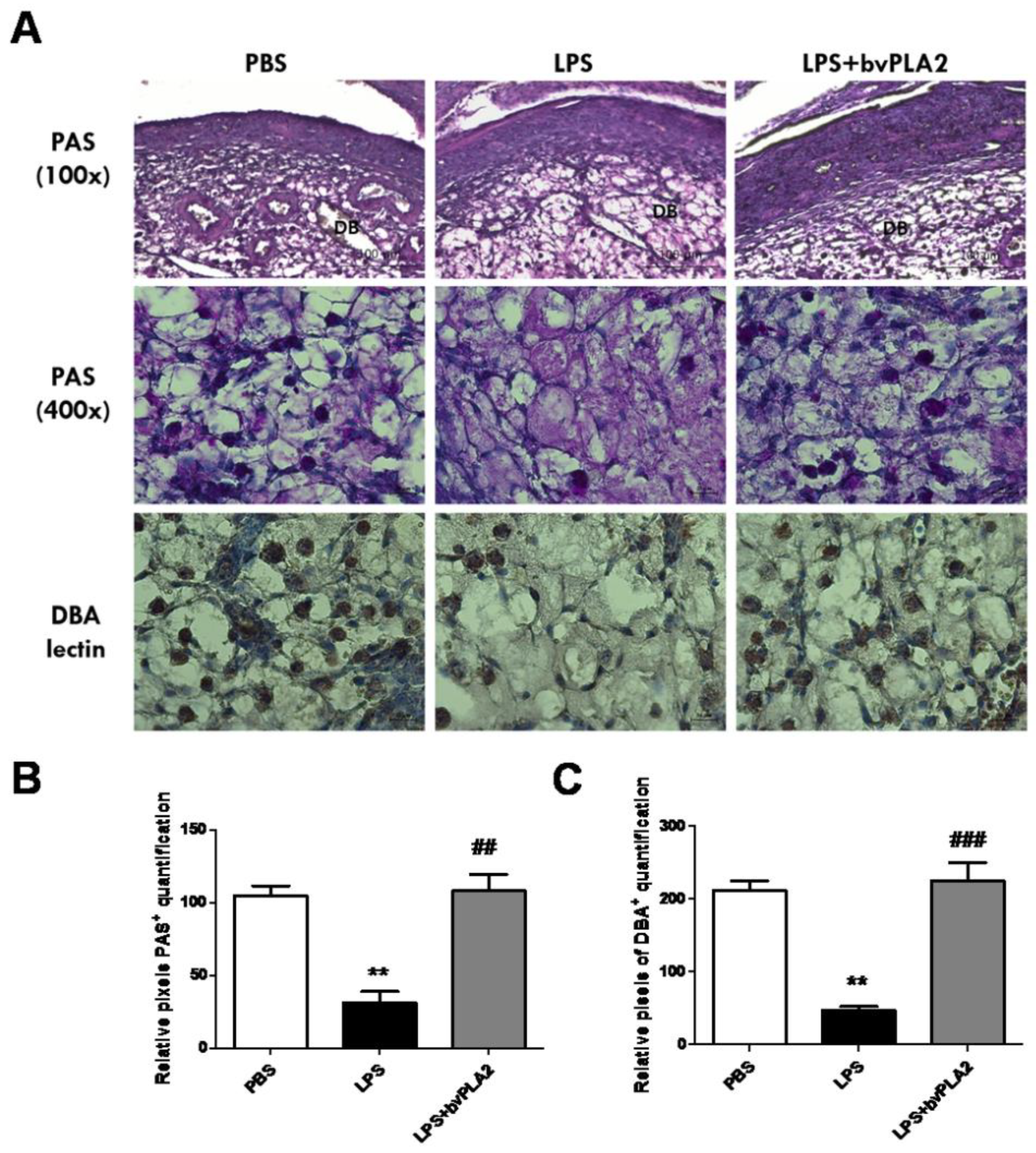
2.5. Effects of bvPLA2 Treatment on uNK Cells in the Decidua of LPS-Induced Abortion Mice
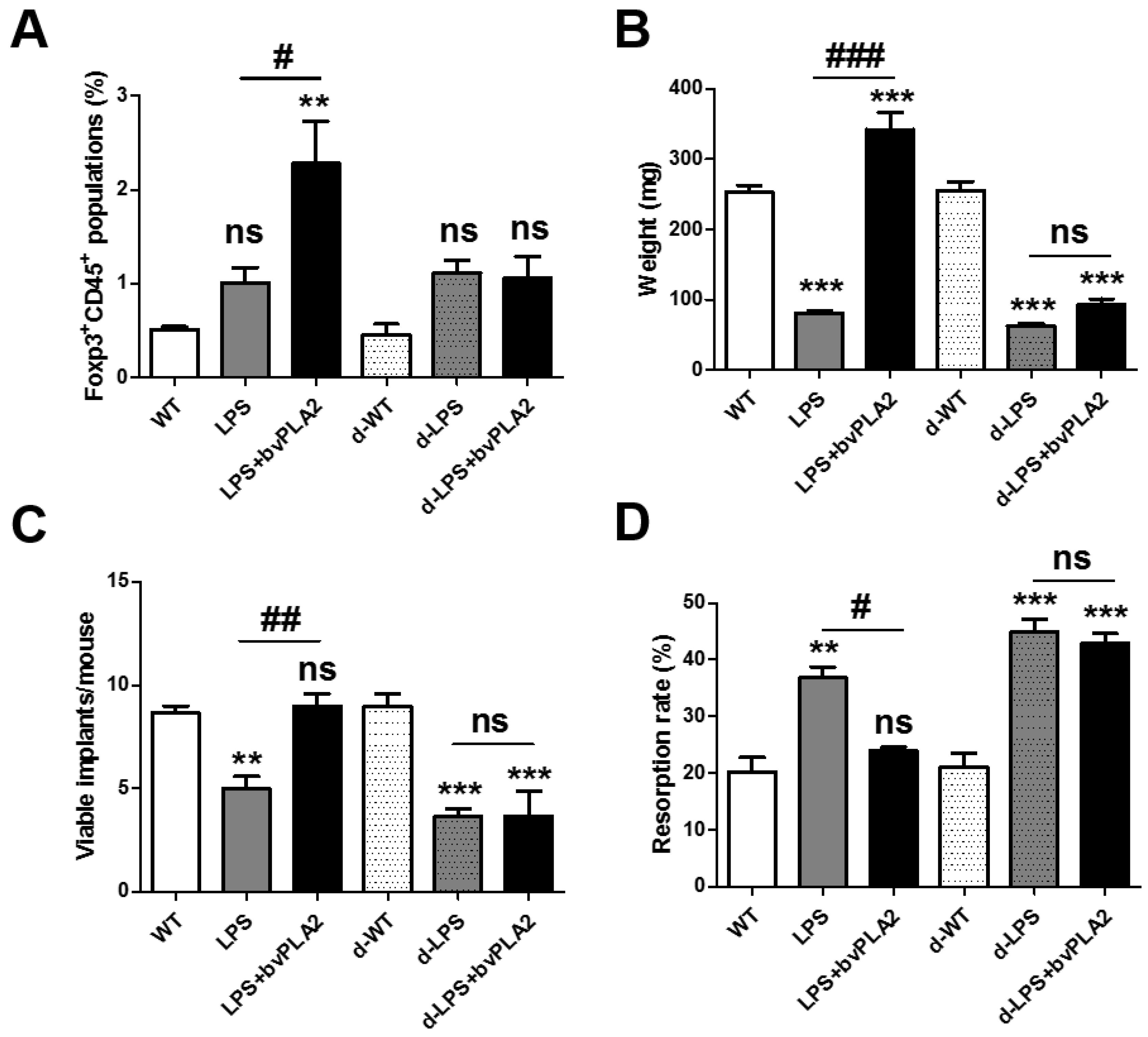
2.6. Effects of Treg Depletion on Fetal Loss in LPS-Induced Abortion Mice Exposed to bvPLA2
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. LPS-Induced Abortion Mouse Model
4.3. Flow Cytometric Analysis
4.4. Measurement of Serum Cytokine Levels
4.5. Real-Time PCR
4.6. ELISA Detection for TNF-α
4.7. Histology Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Thellin, O.; Coumans, B.; Zorzi, W.; Igout, A.; Heinen, E. Tolerance to the foeto-placental ‘graft’: Ten ways to support a child for nine months. Curr. Opin. Immunol. 2000, 12, 731–737. [Google Scholar] [CrossRef]
- Trowsdale, J.; Betz, A.G. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006, 7, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; Chantakru, S.; Esadeg, S.; Ashkar, A.A.; Wei, Q. Decidual natural killer cells: Key regulators of placental development (a review). J. Reprod. Immunol. 2002, 57, 151–168. [Google Scholar] [CrossRef]
- Regan, L.; Rai, R. Epidemiology and the medical causes of miscarriage. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, M.; Leschot, N.J. Genetic aspects of miscarriage. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 855–865. [Google Scholar] [CrossRef]
- Sierra, S.; Stephenson, M. Genetics of recurrent pregnancy loss. Semin. Reprod. Med. 2006, 24, 17–24. [Google Scholar] [CrossRef]
- Amarante-Paffaro, A.M.; Hoshida, M.S.; Yokota, S.; Goncalves, C.R.; Joazeiro, P.P.; Bevilacqua, E.; Yamada, A.T. Localization of cathepsins D and B at the maternal-fetal interface and the invasiveness of the trophoblast during the postimplantation period in the mouse. Cells Tissues Organs 2011, 193, 417–425. [Google Scholar] [CrossRef]
- Christiansen, O.B. Reproductive immunology. Mol. Immunol. 2013, 55, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Kwak-Kim, J.; Yang, K.M.; Gilman-Sachs, A. Recurrent pregnancy loss: A disease of inflammation and coagulation. J. Obstet. Gynaecol. Res. 2009, 35, 609–622. [Google Scholar] [CrossRef]
- Clark, D.A. The importance of being a regulatory T cell in pregnancy. J. Reprod. Immunol. 2016, 116, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004, 10, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.A.; Rahmati, M.; Gohner, C.; Bensussan, A.; Markert, U.R.; Chaouat, G. Seminal plasma peptides may determine maternal immune response that alters success or failure of pregnancy in the abortion-prone CBAxDBA/2 model. J. Reprod. Immunol. 2013, 99, 46–53. [Google Scholar] [CrossRef]
- Teles, A.; Schumacher, A.; Kuhnle, M.C.; Linzke, N.; Thuere, C.; Reichardt, P.; Tadokoro, C.E.; Hammerling, G.J.; Zenclussen, A.C. Control of uterine microenvironment by foxp3(+) cells facilitates embryo implantation. Front. Immunol. 2013, 4, 158. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Zhang, J.L.; Zheng, H.G.; Liu, F.Y.; Chen, Y. Clinical randomized study of bee-sting therapy for rheumatoid arthritis. Zhen Ci Yan Jiu 2008, 33, 197–200. [Google Scholar] [PubMed]
- Kim, H.; Keum, D.J.; Kwak, J.; Chung, H.S.; Bae, H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Lee, G.; Lee, C.; Ye, M.; Chung, H.S.; Kim, H.; Bae, S.J.; Hwang, D.S.; Bae, H. Bee Venom Phospholipase A2, a Novel Foxp3+ Regulatory T Cell Inducer, Protects Dopaminergic Neurons by Modulating Neuroinflammatory Responses in a Mouse Model of Parkinson’s Disease. J. Immunol. 2015, 195, 4853–4860. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Hatta, K.; Lima, P.D.; Yadi, H.; Colucci, F.; Yamada, A.T.; Croy, B.A. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol. Reprod. 2012, 87, 81. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yamada, A.T.; Croy, B.A. DBA-lectin reactivity defines natural killer cells that have homed to mouse decidua. Placenta 2009, 30, 968–973. [Google Scholar] [CrossRef]
- Lahl, K.; Sparwasser, T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol. Biol. 2011, 707, 157–172. [Google Scholar] [CrossRef]
- Christiaansen, A.F.; Boggiatto, P.M.; Varga, S.M. Limitations of Foxp3(+) Treg depletion following viral infection in DEREG mice. J. Immunol. Methods 2014, 406, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.M.; Ramsey, P.S. Progestogen for preventing miscarriage. Cochrane Database Syst. Rev. 2013, 10, 1465–1858. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Farquharson, R.G.; Christiansen, O.B.; Exalto, N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum. Reprod. 2006, 21, 2216–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, P.G.; Goddijn, M.; Middeldorp, S. Antithrombotic therapy for pregnancy loss. Hum. Reprod. Update 2013, 19, 656–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Check, J.H. The use of heparin for preventing miscarriage. Am. J. Reprod. Immunol. 2012, 67, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.L.; Nunes, A.K.; Oliveira, A.G.; Araujo, S.M.; Lemos, A.J.; Rocha, S.W.; Croy, B.A.; Peixoto, C.A. Sildenafil (Viagra(R)) blocks inflammatory injury in LPS-induced mouse abortion: A potential prophylactic treatment against acute pregnancy loss? Placenta 2015, 36, 1122–1129. [Google Scholar] [CrossRef]
- Chaouat, G.; Menu, E.; Clark, D.A.; Dy, M.; Minkowski, M.; Wegmann, T.G. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J. Reprod. Fertil. 1990, 89, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.A. Controversies in reproductive immunology. Crit. Rev. Immunol. 1991, 11, 215–247. [Google Scholar]
- Gendron, R.L.; Nestel, F.P.; Lapp, W.S.; Baines, M.G. Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumour necrosis factor-alpha. J. Reprod. Fertil. 1990, 90, 395–402. [Google Scholar] [CrossRef]
- Clark, D.A.; Chaouat, G.; Arck, P.C.; Mittruecker, H.W.; Levy, G.A. Cytokine-dependent abortion in CBA x DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase [correction of prothombinase]. J. Immunol. 1998, 160, 545–549. [Google Scholar]
- Daher, S.; Fonseca, F.; Ribeiro, O.G.; Musatti, C.C.; Gerbase-DeLima, M. Tumor necrosis factor during pregnancy and at the onset of labor and spontaneous abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 83, 77–79. [Google Scholar] [CrossRef]
- Tangri, S.; Raghupathy, R. Expression of cytokines in placentas of mice undergoing immunologically mediated spontaneous fetal resorptions. Biol. Reprod. 1993, 49, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N. Human endometrial lymphocytes in normal pregnancy and pregnancy loss. Ann. N. Y. Acad. Sci. 1994, 734, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; van den Heuvel, M.J.; Borzychowski, A.M.; Tayade, C. Uterine natural killer cells: A specialized differentiation regulated by ovarian hormones. Immunol. Rev. 2006, 214, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Beaman, K.D.; Gilman-Sachs, A.; Ruiz, J.E.; Schewitz, D.; Beer, A.E. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 1995, 34, 93–99. [Google Scholar] [CrossRef]
- Emmer, P.M.; Nelen, W.L.; Steegers, E.A.; Hendriks, J.C.; Veerhoek, M.; Joosten, I. Peripheral natural killer cytotoxicity and CD56(pos)CD16(pos) cells increase during early pregnancy in women with a history of recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 1163–1169. [Google Scholar] [CrossRef]
- Zavan, B.; do Amarante-Paffaro, A.M.; Paffaro, V.A., Jr. Alpha-actin down regulation and perforin loss in uterine natural killer cells from LPS-treated pregnant mice. Physiol. Res. 2015, 64, 427–432. [Google Scholar]
- Guimond, M.J.; Wang, B.; Croy, B.A. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J. Exp. Med. 1998, 187, 217–223. [Google Scholar] [CrossRef]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; Saito, S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef]
- Tenorio, E.P.; Fernandez, J.; Olguin, J.E.; Saavedra, R. Depletion with PC61 mAb before Toxoplasma gondii infection eliminates mainly Tregs in BALB/c mice, but activated cells in C57BL/6J mice. FEMS Immunol. Med. Microbiol. 2011, 62, 362–367. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, H.; Yang, H.; Lee, J.H.; Kang, N.-H.; Lee, J.; Bae, H.; Hwang, D.-S. Prophylactic Effects of Bee Venom Phospholipase A2 in Lipopolysaccharide-Induced Pregnancy Loss. Toxins 2019, 11, 404. https://doi.org/10.3390/toxins11070404
Baek H, Yang H, Lee JH, Kang N-H, Lee J, Bae H, Hwang D-S. Prophylactic Effects of Bee Venom Phospholipase A2 in Lipopolysaccharide-Induced Pregnancy Loss. Toxins. 2019; 11(7):404. https://doi.org/10.3390/toxins11070404
Chicago/Turabian StyleBaek, Hyunjung, HyeJin Yang, Jong Hoon Lee, Na-Hoon Kang, Jinwook Lee, Hyunsu Bae, and Deok-Sang Hwang. 2019. "Prophylactic Effects of Bee Venom Phospholipase A2 in Lipopolysaccharide-Induced Pregnancy Loss" Toxins 11, no. 7: 404. https://doi.org/10.3390/toxins11070404




