1. Introduction
Milling processes are methods that can be used to transform whole grains into forms suitable for conversion into consumable products. They usually separate the botanical tissue of the grain and reduce the endosperm into flour or grits [
1].
From the processing perspective, the maize kernel is composed of four primary structures: the endosperm, germ, pericarp and tip cap, and they generally make up 83%, 11%, 5% and 1% of the maize kernel, respectively [
2]. The endosperm is mainly made up of starch surrounded by a protein matrix. There are two types of endosperm and these influence grain hardness: floury (soft or mealy) or horny (hard or vitreous), which depend on the size, morphology and compaction grade of the starch granules and the nature of the protein matrix. The type of proteins in particular are the determining factor that can explain endosperm vitreousness [
3].
Most of the maize used for food is first processed by either wet or dry milling industries. Wet milling fundamentally differs from dry milling in that it is a maceration process in which physical and chemical changes occur in the nature of the basic constituents. Wet milling produces pure starch for industrial and food uses, and by-products composed of protein, fibre and germ [
1].
Dry-milling is the main milling procedure adopted in the maize food chain, and it produces refined endosperm products with various particle sizes and other by-products such as germ and animal feed flour [
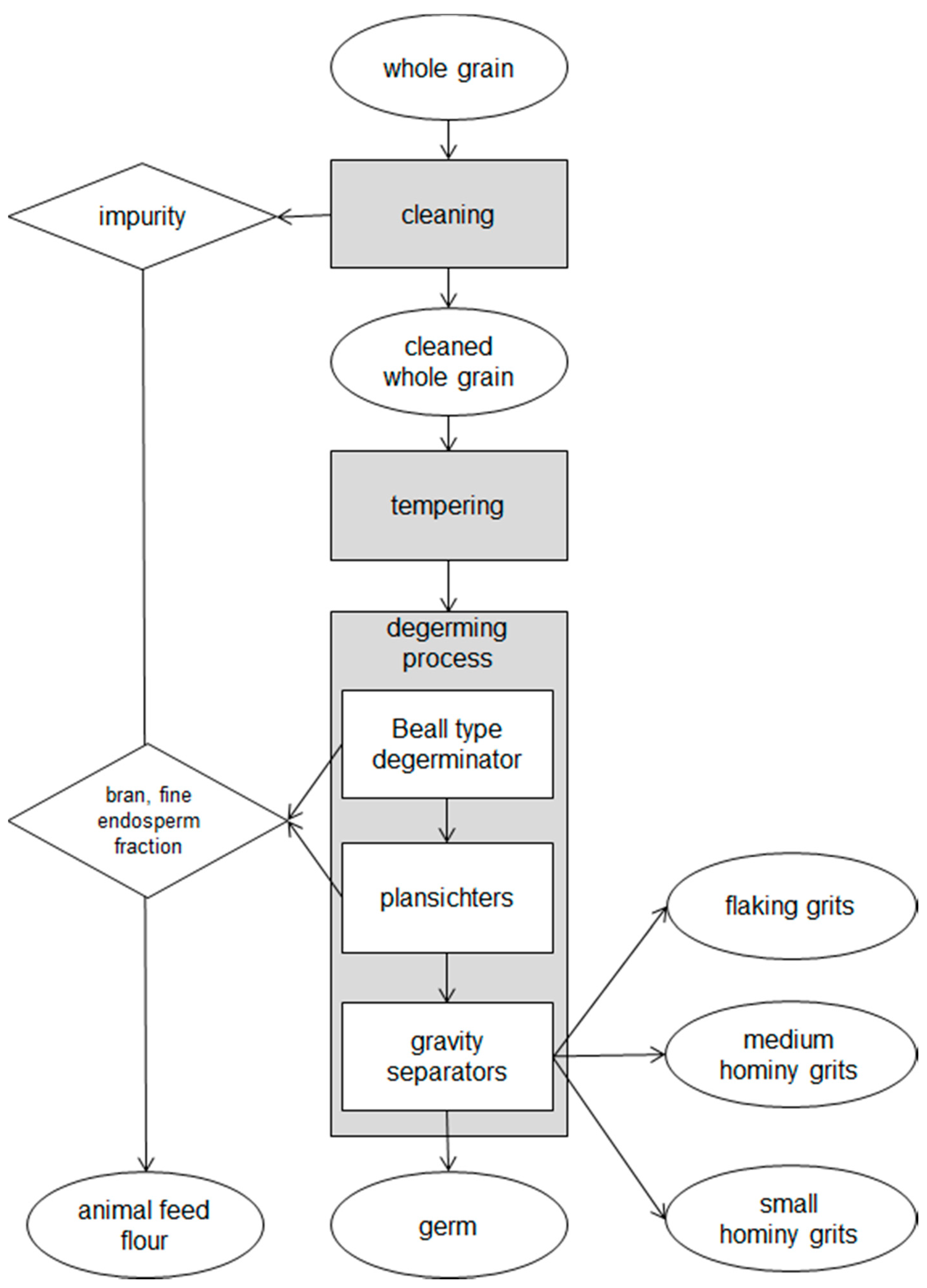
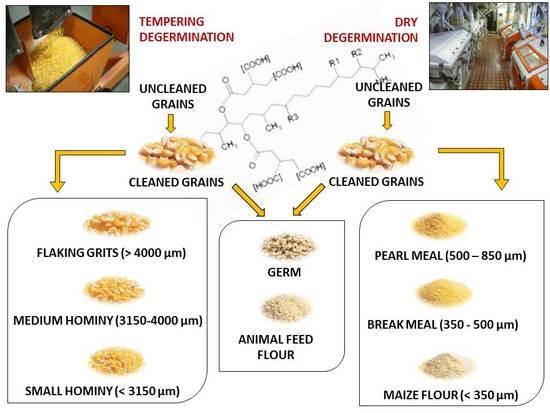
4]. This process could be carried out with tempering-degermination (TD) or without dry-degermination (DD) a tempering step before degermination. Tempering is done adding water in order to create differential swelling resulting from the higher absorbing moisture of germ and pericarp, that lead to a loss of tissue connection between the germ and the endosperm and a faster removal of these fractions [
5]. The heart of the TD process is a Beall-type degerminator that produce a high yield of large particle size grits (flaking grits, hominy grits) with low-fat content, suitable for manufacture of corn flakes and other extruded transformations [
4], although further grinding and refining processes could be applied to these products in order to obtain maize meal and flour. Otherwise, in the DD system, maize grains are broken by an impact degerminator, and this process is employed only for the production of maize meal and flour. High kernel hardness [
6], with high incidence of horny endosperm and low incidence of stress cracks [
4], related mainly to high temperature during the drying process, are the major quality criteria for maize used in dry milling, in particular for TD.
Considering the increasing role of maize milled products as basic food, their quality characterization becomes extremely important. From a nutritional point of view, maize and its derived products are good sources of starch, proteins, lipids and different bioactive compounds [
7,
8]. However, the quality of maize could be reduced by the presence of mycotoxins and in particular by B-series fumonisins (FBs), a class of secondary fungal metabolites produced by
Fusarium species from the
Liseola section that are the most important mycotoxins found in maize grain in temperate areas [
9,
10]. As FB producer fungi grow over a wide range of temperatures, but only when there is a high water activity (a
w > 0.9), these mycotoxins are formed in maize prior to the harvest. B-series fumonisins are fairly heat-stable and their content is only significantly reduced during processes in which the temperature exceeds 150 °C [
9]. Even when there is no detoxification, a re-distribution of FBs has been observed in milled products, with a very different content of these metabolites in the kernel components [
11]. The mycotoxins may be distributed during the milling process, thus yielding higher or lower levels in the various milled products [
12]. The control of the FBs content in the chain from the unprocessed grains to the processed maize-based foods is necessary considering also the European Commission Regulation (EC) No. 1126/2007 [
13].
The re-distribution of FBs in maize dry milling has already been analysed extensively in other studies [
14,
15,
16,
17], but always considering separately a DD system or a TD degermination system. The aim of this study has been to investigate and compare the distribution of fumonisin B
1 (FB
1), fumonisin B
2 (FB
2) and FBs as a sum of FB
1 and FB
2 in milling products and by-products obtained from two degermination systems and from the processing of the same maize lots at the industrial commercial scale, in order to obtain a clear comparison of the decontamination levels obtainable by applying different dry-milling processes.
2. Results and Discussion
As far as the considered growing seasons are concerned, the average recorded FB contamination in pre-cleaned whole grain was 357 µg·kg
−1, 1292 µg·kg
−1, 2123 µg·kg
−1, in 2011, 2012 and 2013 growing seasons, respectively (
Table 1).
Analysis of variance (ANOVA) showed significant differences (p < 0.001) for both FB1 and FB2 and for their sum, between the milling fractions obtained considering both the compared dry-milling processes. Otherwise the interaction between the milling fraction and the year of production of the processed lots was never significant for FB contamination.
The effect of the cleaning operation on the FB concentration was significant, with a mean content in the pre-cleaned and post-cleaned whole grain of 1257 µg·kg
−1 and 725 µg·kg
−1, respectively, which corresponds to a content reduction of 42%. The abatement recorded in this production situation was on average higher than that observed for FB in previous studies with different cleaning steps (−10; −32% [
18]; −32% [
19]).
It is important to note that each commercial mill is likely to have its own particular set up, thus giving rise to different percentages of reduction, but a cleaning process is essential to obtain a healthy whole grain for milling.
The evaluation of the distribution of FBs in the maize kernel can start from the two botanical tissues that are usually separated at the beginning of the milling process: the germ and the bran. Even though two different degermination processes were considered, the effect on the FB contamination of the germ was the same. The mean FB content was 528 µg·kg
−1 and 510 µg·kg
−1 in the DD system and in the TD system, respectively. Germ contamination was not significantly different from that of the whole grain after cleaning (
Table 2).
The animal feed flour, a mixture of bran and part of the mealy endosperm, was the by-product with the highest FB content (2.2 times higher in the DD process and 2.4 times in the TD process than the whole grain before cleaning). No significant differences were observed for the animal feed flour contamination of the compared processes.
Several previous scientific studies collected and analysed germ and animal feed flour together, and the results showed a 2 to 3 times higher FB content than the whole grain [
15,
20,
21]. In other scientific studies [
16,
17], in the same way as in the present study, the germ was collected separately from the bran or the fine particles destined for animal consumption and it was always contaminated less than the pre-cleaned whole grain (−30% and −40%, respectively), while the animal feed flour was the highest contaminated fraction.
As a consequence of this unequal distribution of mycotoxins in the milling fractions, the products destined for human consumption were always significantly (p < 0.001) less contaminated than the germ and animal feed flour, and showed a variable re-distribution from the whole grain before cleaning in the different compared processes. The pearl meal, break meal and maize flour from the DD process had mean FB contents of 157, 216 and 623 µg·kg−1, respectively, which resulted in a decontamination, compared to the whole kernel before cleaning, of 87%, 83% and 50%, respectively. As far as the particle size effect is concerned, the FB content in the pearl and break meal was significantly lower (p < 0.001) than in the maize flour.
Considering the TD system, the mean FB content of the flaking grits and of the medium and small hominy grits was 76, 147 and 272 µg·kg−1, respectively. The FB reduction, compared to the whole grain before cleaning, was 94%, 88% and 78%, respectively. These fractions with different particle sizes significantly differed from each other as far as the FB content was concerned: compared to the flaking grits, the contamination was 1.9 and 3.6 times higher for the medium and small hominy grits, respectively.
From a comparison of the applied degermination processes, it is possible to state that the TD system achieved the best FB decontamination results for the flaking grits, which were significantly less contaminated: considering the percentage of reduction with respect to the whole grain before cleaning, the flaking grits showed 6% of the original kernel FB content, while the pearl meal and break meal instead showed a FB contamination that ranged from 12% to 17% with respect to the whole grain. When the single compounds, that is FB1 and FB2, were considered, the same percentage of reduction as that of the FBs was observed.
The milling yields and FB mass balance of each fraction are reported in
Table 3 in order to facilitate the comparison of the considered systems. The mass balance, calculated for each fraction from the combination of the yield data during the milling process and the FB content, is a parameter able to describe how and in what percentage the mycotoxin is distributed in the fraction derived from maize kernel milling.
Considering the sum of the three products for human consumption obtained through TD process (flaking grits and medium and small hominy grits) the mean milling yield was 55%, while they only contributed to 6% of the total FB contamination of the whole grain. The pearl meal, break meal and maize flour from the DD dry milling system had a mean yield of 55%, and their FB content amounted to 9% of the total contamination of the kernel. These three products mainly originate from the horny endosperm, but a higher percentage of floury endosperm that is not completely separated by this process still remains especially in the maize flour [
11].
Previous studies have overlooked the fumonisin redistribution with different milling process, whereas the present study has been specifically designed to directly compare the decontamination of FBs obtained through the application of different degermination and milling processes to the same lots of maize grain.
To compare the results of several scientific studies concerning the effect of maize milling on the distribution of mycotoxins,
Table 4 summarizes the results of different degermination processes, under both experimental and industrial dry-milling conditions. The data are expressed as the percentage of FB remaining in each of the degermination milling fractions compared to the contamination of the whole kernel. All the milling fractions destined for human consumption are reported according to the description adopted by the authors, and when available, the particle size of each fraction is reported. All the studies pertaining to both of the considered degermination systems confirm the negative relationship between the particle size and the fumonisin content. The FB contamination of flaking grits derived from the TD processes ranges between 6 and 15%, compared to that of whole kernel. In the considered DD processes, the lowest occurrence of FBs was detected in the pearl and break meal and varied between 11 and 22%, compared to that of whole kernel. A reduction in the FB content of the maize flour was also observed, with respect to the whole grain, but the decontamination percentage fell between 40–60%.
Castelles et al. [
21] attributed the high contamination rates in maize flour to the fact that this fraction is obtained from the fine particles that are mainly created during the milling of the external kernel layers. Other authors [
16,
20] instead considered the hypothesis that these high rates may be due to contamination of the flour with germ particles. Kent and Evers [
1] and Gwirtz and Garcia-Casal [
2] reported a negative relationship between the particle size of maize dry-milled fractions and their fat content. Thus, according to these authors, the main contribution of FB contamination to maize flour could originate from the floury endosperm located around the germ, where a higher development of fungal species is possible. Katta et al. [
16] suggested that the fungus could primarily be located in the pedicel and germ, but Shim et al. [
22] showed, through experimental data, that
F. verticillioides produces only a few FBs in the germ. Therefore, even though the germ is not the best substrate for FB production, the floury endosperm around the germ could instead be a good place for the fungus that grows in the germ to produce mycotoxins. Philippeau et al. [
23] reported that the starch in a vitreous endosperm is more encapsulated by prolamin (zein) proteins than in a floury endosperm, thus vitreous particles of maize kernels may be more resistant to microbial attack than floury ones.
Moreover, considering the amylose/amylopectin ratio of the maize endosperm, Dombrink-Kurtzman and Knutson [
24] concluded that there is a higher amylopectin content in the soft endosperm than in the hard endosperm. Bluhm and Woloshuk [
25] observed that the occurrence of amylopectin during kernel development induces FB
1 biosynthesis, possibly through the uptake of α-1,6 linked glucosides, such as dextrin. Field experiments conducted in Italy [
26] showed that waxy maize hybrids, which are basically constituted by amylopectin, were more contaminated with fumonisin than conventional hybrids.
Overall, the collected data underline how the floury endosperm was more contaminated than the horny one, and that the different effectiveness of degermination systems to separate these fractions could lead to a different decontamination capacity in the derived products. Thus, the TD system process, which is able to better separate the horny endosperm from finer fractions [
1], permits a higher decontamination level to be obtained than the DD system. It is important to underline that several industrial mills, including the one considered in this study, apply a TD system with a Beall degerminator to produce grits that are subsequently refined into pearl and break meal and maize flour. In this case, the use of TD systems is not aimed at obtaining flaking grits, but at increasing the decontamination level in the derived meal and flour fractions.
Considering European legislation regarding fumonisins (sum of FB
1 and FB
2) [
13], different maximum levels are set for smaller and larger milling fractions than 500 µm; these levels reflect the contamination level of the different fractions and are 2000 and 1400 µg kg
−1, respectively. All the samples collected in this study were under the regulatory levels recommended.