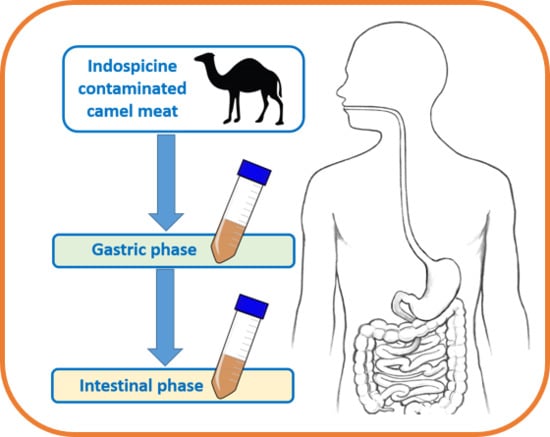
Release of Indospicine from Contaminated Camel Meat following Cooking and Simulated Gastrointestinal Digestion: Implications for Human Consumption
Abstract
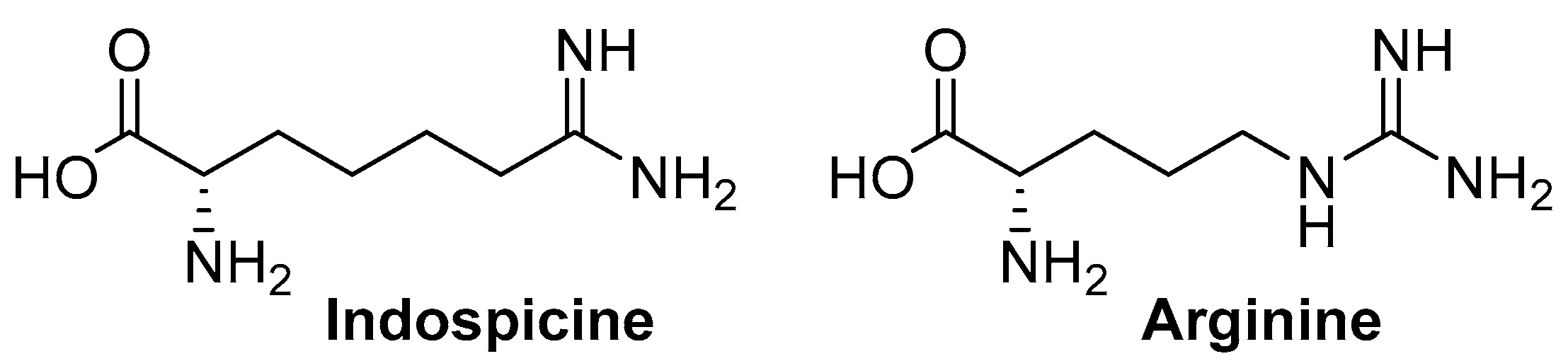
:1. Introduction
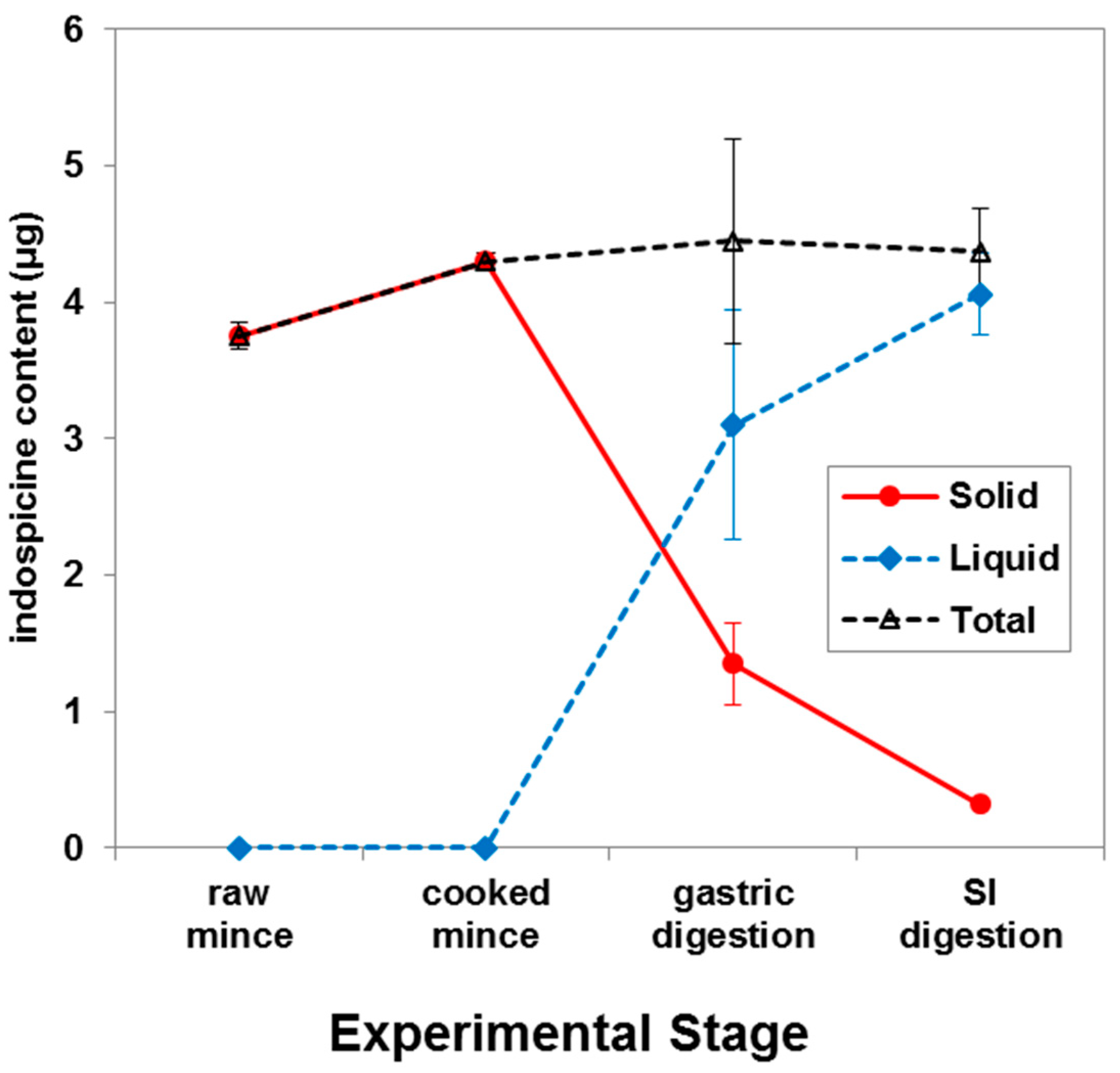
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Study Design
4.3. Camel Meat Samples
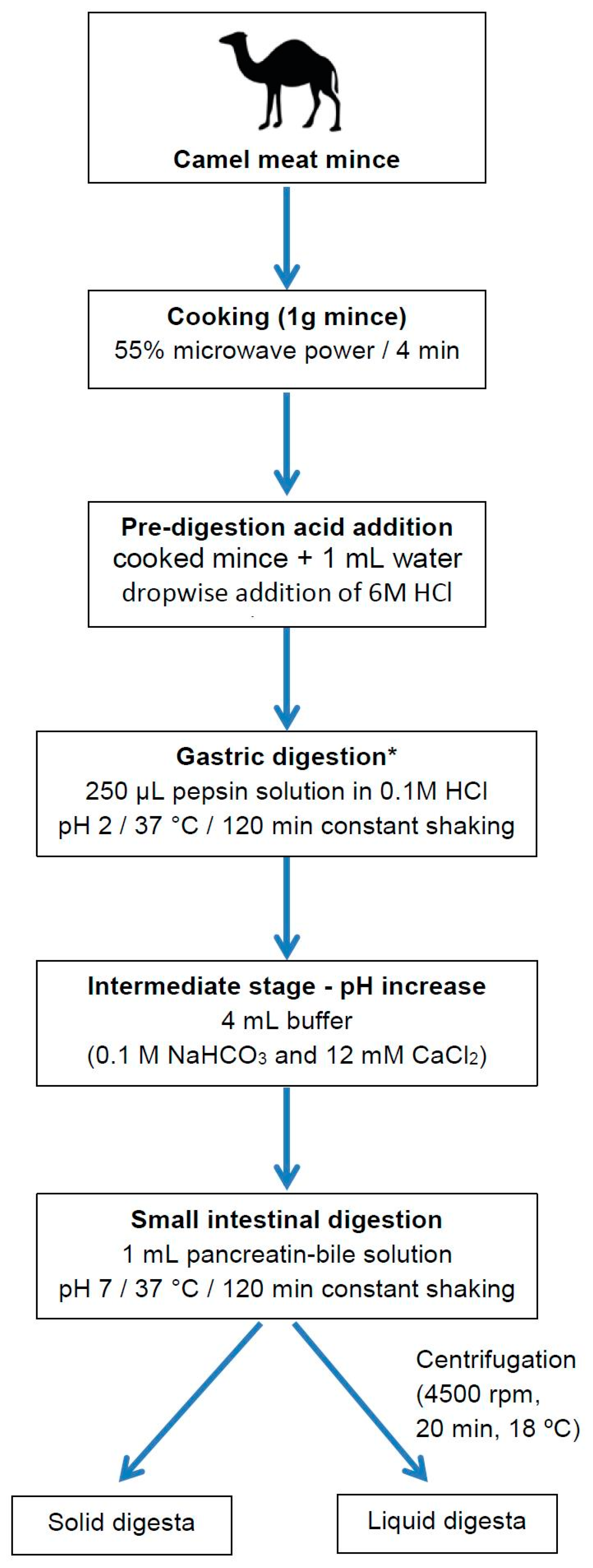
4.4. In Vitro Digestion of Camel Meat
4.4.1. Gastric Digestion
4.4.2. Small Intestine Digestion
4.4.3. Separation of Liquid and Solid Digesta
4.5. Preparation of External and Internal Standards for LC-MS/MS Analysis
4.6. Extraction of Indospicine from Camel Meat Samples and Digesta
4.7. LC-MS/MS Analysis of Samples
4.8. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Tan, E.T.T.; Materne, C.M.; Silcock, R.G.; D’Arcy, B.R.; Al Jassim, R.; Fletcher, M.T. Seasonal and species variation of the hepatotoxin indospicine in Australian Indigofera legumes as measured by UPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 6613–6621. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.T.; Al Jassim, R.A.M.; Cawdell-Smith, A.J. The occurrence and toxicity of indospicine to grazing animals. Agriculture 2015, 5, 427–440. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Reichmann, K.G.; Ossedryver, S.M.; McKenzie, R.A.; Carter, P.D.; Blaney, B.J. Accumulation and depletion of indospicine in calves (Bos taurus) fed creeping indigo (Indigofera spicata). Anim. Prod. Sci. 2018, 58, 568–576. [Google Scholar] [CrossRef]
- Tan, E.T.T.; Al Jassim, R.; Cawdell-Smith, A.J.; Ossedryver, S.M.; D’Arcy, B.R.; Fletcher, M.T. Accumulation, persistence, and effects of indospicine residues in camels fed Indigofera plant. J. Agric. Food Chem. 2016, 64, 6622–6629. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, M.P.; Kelly, W.R.; McEwan, D.; Williams, O.J.; Cameron, R. Hepatotoxicity to dogs of horse meat contaminated with indospicine. Aust. Vet. J. 1988, 65, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Christie, G.S.; Madsen, N.P.; Hegarty, M.P. Acute biochemical changes in rat liver induced by the naturally-occurring amino acid indospicine. Biochem. Pharmacol. 1969, 18, 693–700. [Google Scholar] [CrossRef]
- Nordfeldt, S.; Henke, L.A.; Morita, K.; Matsumoto, H.; Takahash, M.; Younge, O.R.; Willers, E.H.; Cross, R.F. Feeding tests with Indigofera endecaphylla Jacq. (Creeping indigo) and some observations on its poisonous effects on domestic animals. Univ. Hawaii Agric. Exp. Stat. Technol. Bull. 1952, 15, 5–23. [Google Scholar]
- Ossedryver, S.M.; Baldwin, G.I.; Stone, B.M.; McKenzie, R.A.; Eps, A.W.; Murray, S.; Fletcher, M.T. Indigofera spicata (creeping indigo) poisoning of three ponies. Aust. Vet. J. 2013, 91, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Madsen, N.P.; Christie, G.S.; Hegarty, M.P. Effect of indospicine on incorporation of L-arginine-14C into protein and transfer ribonucleic acid by cell-free systems from rat liver. Biochem. Pharmacol. 1970, 19, 853–857. [Google Scholar] [CrossRef]
- Christie, G.S.; De Munk, F.G.; Madsen, N.P.; Hegarty, M.P. Effects of an arginine antagonist on stimulated human lymphocytes in culture. Pathology 1971, 3, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, M.P.; Pound, A.W. Indospicine, a hepatotoxic amino acid from Indigofera spicata: Isolation, structure, and biological studies. Aust. J. Biol. Sci. 1970, 23, 831–842. [Google Scholar] [CrossRef]
- Pearn, J.H.; Hegarty, M.P. Indospicine—The teratogenic factor from Indigofera spicata extract causing cleft palate. Br. J. Exp. Pathol. 1970, 51, 34–36. [Google Scholar] [PubMed]
- Christie, G.S.; Wilson, M.; Hegarty, M.P. Effects on the liver in the rat of ingestion of Indigofera spicata, a legume containing an inhibitor of arginine metabolism. J. Pathol. 1975, 117, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Hutton, E.M.; Windrum, G.M.; Kratzing, C.C. Studies on the toxicity of Indigofera endecaphylla: II. Toxicity for mice. J. Nutr. 1958, 65, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hutton, E.M.; Windrum, G.M.; Kratzing, C.C. Studies on the toxicity of Indigofera endecaphylla: I. Toxicity for rabbits. J. Nutr. 1958, 64, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.R.; Young, M.P.; Hegarty, M.P.; Simpson, G.D. The hepatotoxicity of indospicine in dogs. In Poisonous Plants; James, L.F., Keeler, R.F., Bailey, E.M., Cheeke, P.R., Hegarty, M.P., Eds.; Iowa State University Press: Ames, IA, USA, 1992; pp. 126–130. [Google Scholar]
- FitzGerald, L.M.; Fletcher, M.T.; Paul, A.E.; Mansfield, C.S.; O’Hara, A.J. Hepatotoxicosis in dogs consuming a diet of camel meat contaminated with indospicine. Aust. Vet. J. 2011, 89, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.T.T.; Al Jassim, R.; D’Arcy, B.R.; Fletcher, M.T. In vitro biodegradation of hepatotoxic indospicine in Indigofera spicata and its degradation derivatives by camel foregut and cattle rumen fluids. J. Agric. Food Chem. 2017, 65, 7528–7534. [Google Scholar] [CrossRef] [PubMed]
- Bobrich, A.; Fanning, K.J.; Rychlik, M.; Russell, D.; Topp, B.; Netzel, M. Phytochemicals in Japanese plums: Impact of maturity and bioaccessibility. Food Res. Int. 2014, 65, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Braga, A.C.; Alves, R.N.; Maulvault, A.L.; Barbosa, V.; Marques, A.; Costa, P.R. In vitro bioaccessibility of the marine biotoxin okadaic acid in shellfish. Food Chem. Toxicol. 2016, 89, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ekbatan, S.S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Versantvoort, C.H.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.; Sips, A.J. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, M.P. Non-metallic chemical residues in toxic plants with potential importance to animal and human health. In Vet Update ‘92’; Osborne, H.G., Ed.; University of Queensland. Continuing Professional Education: Brisbane, Australia, 1992; pp. 323–332. [Google Scholar]
- Pollitt, S.; Hegarty, M.P.; Pass, M.A. Analysis of the amino acid indospicine in biological samples by high performance liquid chromatography. Nat. Toxins 1999, 7, 233–240. [Google Scholar] [CrossRef]
- Tan, E.T.T.; Al Jassim, R.; D’Arcy, B.R.; Fletcher, M.T. Level of natural hepatotoxin (Indospicine) contamination in Australian camel meat. Food Addit. Contam. Part A 2016, 33, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.; Clarke, M.; Lethbridge, M.; Sobels, J. Central Australian Commercial Camel Meat Viability Study, Report to the Northern Territory and South Australian Governments, 2015–2016. 71p; Agriknowledge: Mylor, South Australia, 2016; Available online: https://agriknowledge2.weebly.com/agribusiness.html (accessed on 20 August 2018).
- enHealth Council. Environmental Health Risk Assessment: Guidelines for Assessing Human Health Risks from Environmental Hazards. 2012. Available online: http://www.eh.org.au/documents/item/916 (accessed on 2 August 2018).
- ABS. Australian Health Survey: Nutrition First Results—Foods and Nutrients, 2011-12. 2014. Available online: http://www.abs.gov.au/ausstats/[email protected]/detailspage/4364.0.55.0072011-12 (accessed on 2 August 2018).
- Sultan, S.; Osborne, S.A.; Addepalli, R.; Netzel, G.; Netzel, M.E.; Fletcher, M.T. Indospicine cytotoxicity and transport in human cell lines. Food Chem. 2018, 267, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Aylward, J.; Haydock, K.; Strickland, R.; Hegarty, M. Indigofera species with agronomic potential in the tropics. Rat toxicity studies. Crop Pasture Sci. 1987, 38, 177–186. [Google Scholar] [CrossRef]
- Gardner, D.R.; Riet-Correa, F. Analysis of the toxic amino acid indospicine by liquid chromatography-tandem mass spectrometry. Int. J. Poisonous Plant Res. 2011, 1, 20–27. [Google Scholar]
- Tan, E.T.T.; Fletcher, M.T.; Yong, K.W.L.; D’Arcy, B.R.; Al Jassim, R. Determination of hepatotoxic indospicine in Australian camel meat by ultra-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Morita, J.-I.; Yasui, T. Involvement of hydrophobic residues in heat-induced gelation of myosin tail subfragments from rabbit skeletal muscle. Agric. Biol. Chem. 1991, 55, 597–599. [Google Scholar]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Zabaras, D.; Lundin, L.; Day, L.; Addepalli, R.; Osborne, S.A.; Seymour, R. Release and absorption of carotenes from processed carrots (Daucus carota) using in vitro digestion coupled with a Caco-2 cell trans-well culture model. Food Res. Int. 2011, 44, 868–874. [Google Scholar] [CrossRef]
- Hegarty, M.P. Toxic amino acids in foods of animals and man. Proc. Nutr. Soc. Aust. 1986, 11, 73–81. [Google Scholar]
- Tan, E.T.T.; Yong, K.W.L.; Wong, S.-H.; D’Arcy, B.R.; Al Jassim, R.; De Voss, J.J.; Fletcher, M.T. Thermo-alkaline treatment as a practical degradation strategy to reduce indospicine contamination in camel meat. J. Agric. Food Chem. 2016, 64, 8447–8453. [Google Scholar] [CrossRef] [PubMed]
- Pass, M.A. Contaminated Horsemeat. Assessment and Prevention of Toxicity from Indospicine. RIRDC Report, Project No UQ-46A; Rural Industries Research & Development Corporation: Kingston, ACT, Australia, 2000; p. 9. [Google Scholar]
- Kong, F.; Singh, R.P. Disintegration of solid foods in human stomach. J. Food Sci. 2008, 73, R67–R80. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Horowitz, M.; Maddox, A.; Myers, J.C.; Chatterton, B.E. Effects of increasing solid component size of a mixed solid/liquid meal on solid and liquid gastric emptying. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 271, G549–G554. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.-S.; Wong, S.-H.; Chow, S.; Challinor, V.L.; Yong, K.W.L.; Fletcher, M.T.; Arthur, D.M.; Ng, J.C.; De Voss, J.J. Synthesis of l-indospicine, [5,5,6-2H3]-l-indospicine and l-norindospicine. Org. Biomol. Chem. 2016, 14, 6826–6832. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, S.; Giles, C.; Netzel, G.; Osborne, S.A.; Netzel, M.E.; Fletcher, M.T. Release of Indospicine from Contaminated Camel Meat following Cooking and Simulated Gastrointestinal Digestion: Implications for Human Consumption. Toxins 2018, 10, 356. https://doi.org/10.3390/toxins10090356
Sultan S, Giles C, Netzel G, Osborne SA, Netzel ME, Fletcher MT. Release of Indospicine from Contaminated Camel Meat following Cooking and Simulated Gastrointestinal Digestion: Implications for Human Consumption. Toxins. 2018; 10(9):356. https://doi.org/10.3390/toxins10090356
Chicago/Turabian StyleSultan, Saira, Cindy Giles, Gabriele Netzel, Simone A. Osborne, Michael E. Netzel, and Mary T. Fletcher. 2018. "Release of Indospicine from Contaminated Camel Meat following Cooking and Simulated Gastrointestinal Digestion: Implications for Human Consumption" Toxins 10, no. 9: 356. https://doi.org/10.3390/toxins10090356