Snake Venoms in Cancer Therapy: Past, Present and Future
Abstract
:1. Introduction
2. Early-Stage Study on Snake Venoms in Cancer Therapy
3. Development of Snake Venoms for Cancer Target Therapy
3.1. Antiangiogenesis
3.2. Apoptosis Induction
4. Future Directions
4.1. Isolation and Characterization of New Active Molecules from Snake Venoms by Snake Venomics
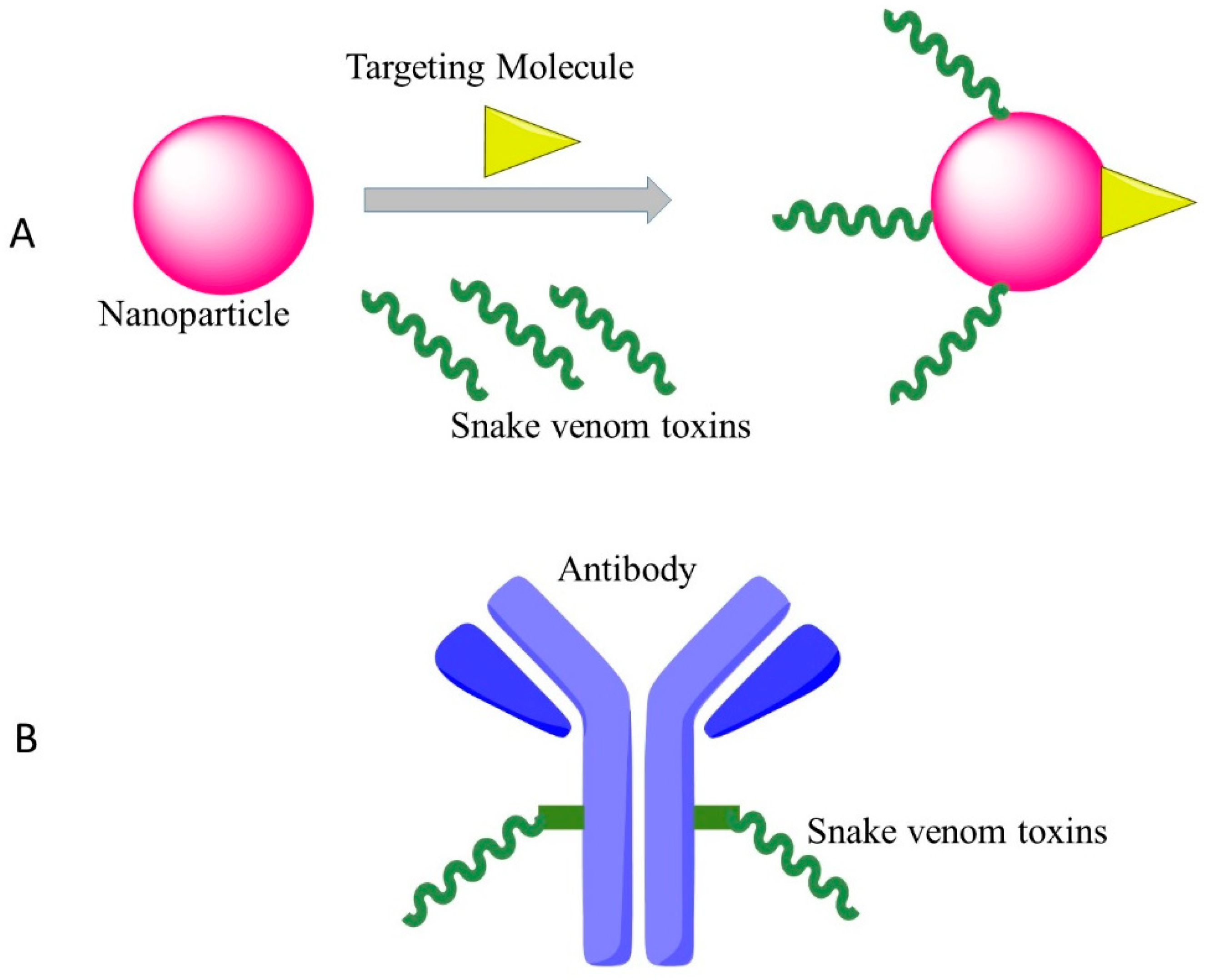
4.2. New Drug Delivery System/Coupled with Monoclonal Antibody
5. Conclusions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer. J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.B. Mechanism-based target identification and drug discovery in cancer research. Science 2000, 287, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Aarti, C.; Khusro, A. Snake venom as anticancer agent-current perspective. Int. J. Pure Appl. Biosci. 2013, 1, 24–29. [Google Scholar]
- Koh, D.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Xu, Z.; Qian, C.; Jiang, F.; Ding, X.; Ruan, Y.; Ding, Z.; Fan, Y. Antiplatelet aggregation and antithrombosis efficiency of peptides in the snake venom of deinagkistrodon acutus: Isolation, identification, and evaluation. Evid. Based Complement. Altern. Med. 2015, 2015, 412841. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Ondetti, M.A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 1991, 17, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Sanhajariya, S.; Duffull, S.; Isbister, G. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Essex, H.E.; Priestley, J.T. Effect of rattlesnake venom on flexner-jobling’s carcinoma in the white rat (mus norvegicus albinus.). Proc. Soc. Exp. Biol. Med. 1931, 28, 550–551. [Google Scholar] [CrossRef]
- Kurotchkin, T.; Spies, J. Effects of cobra venom on the Fujinami rat sarcoma. Proc. Soc. Exp. Biol. Med. 1935, 32, 1408–1410. [Google Scholar] [CrossRef]
- Des Ligneris, M.; Grasset, E. Clinical experiments on the effect of African snake venoms on human cancer cases with or without concomitant deep therapy. Am. J. Cancer 1936, 26, 512–520. [Google Scholar] [CrossRef]
- Drueck, C.J. Cdbra venom and opiates in the pain of cancer of the rectum. Anesth. Analg. 1942, 21, 41–45. [Google Scholar]
- Macht, D.I. Experimental and clinical study of cobra venom as an analgesic. Proc. Natl. Acad. Sci. USA 1936, 22, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.; Sehra, K.; Mukerji, B.; Chopra, R. Choline esterase in cobra venom. Curr. Sci. 1938, 7, 51–53. [Google Scholar]
- Zeller, E. Occurrence and nature of the cholinesterase of snake venoms. Experientia 1947, 3, 375–379. [Google Scholar] [CrossRef]
- Duran-Reynals, F. The invasion of the body by animal poisons. Science 1936, 83, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Zeller, E.; Maritz, A. A new l-amino acid oxidase. Helv. Chim. Acta 1944, 27, 1888–1902. [Google Scholar] [CrossRef]
- Singer, T.P.; Kearney, E.B. The l-amino acid oxidases of snake venom. II. Isolation and characterization of homogeneous l-amino acid oxidase. Arch. Biochem. 1950, 29, 190–209. [Google Scholar] [PubMed]
- Kearney, E.; Singer, T.P. The l-amino acid oxidases of snake venom. III. Reversible inactivation of l-amino acid oxidases. Arch. Biochem. Biophys. 1951, 33, 377–396. [Google Scholar] [CrossRef]
- Kearney, E.; Singer, T.P. The l-amino acid oxidases of snake venom. IV. The effect of anions on the reversible inactivation. Arch. Biochem. Biophys. 1951, 33, 397–413. [Google Scholar] [CrossRef]
- Kearney, E.; Singer, T.P. The l-amino acid oxidases of snake venom. V. Mechanism of the reversible inactivation. Arch. Biochem. Biophys. 1951, 33, 414–426. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Foster, S.; Lyubimov, A.Y.; Vrielink, A. Crystal structure of LAAO from Calloselasma rhodostoma with an l-phenylalanine substrate: Insights into structure and mechanism. J. Mol. Biol. 2006, 364, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Hanahan, D.J. A study of the purification and properties of the phospholipase a of Crotalus adamanteus venom. Biochemistry 1962, 1, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-W.; Tinker, D.O. Phospholipase A2 from Crotalus atrox venom. I. Purification and some properties. Biochemistry 1969, 8, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, C.; Yutani, F.; Kajiyashiki, T. Amino acid sequences of eight phospholipases A2 from the venom of australian king brown snake, Pseudechis australis. Toxicon 1990, 28, 329–339. [Google Scholar] [CrossRef]
- Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999, 9, 585–590. [Google Scholar] [CrossRef]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stocker, K.; Barlow, G.H. The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol. 1976, 45, 214–223. [Google Scholar] [PubMed]
- Kirby, E.P.; Niewiarowski, S.; Stocker, K.; Kettner, C.; Shaw, E.; Brudzynski, T.M. Thrombocytin, a serine protease from Bothrops atrox venom. 1. Purification and characterization of the enzyme. Biochemistry 1979, 18, 3564–3570. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S., Jr.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, J.B.; Tu, A.T. Hemorrhagic toxins from western diamondback rattlesnake (Crotalus atrox) venom: Isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry 1978, 17, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.J.; Polokoff, M.A.; Friedman, P.A.; Huang, T.-F.; Holt, J.C.; Cook, J.J.; Niewiarowski, S. Disintegrins: A family of integrin inhibitory proteins from viper venoms. Proc. Soc. Exp. Biol. Med. 1990, 195, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Huang, T.-F. Potent platelet aggregation inhibitor from Trimeresurus gramineus snake venom. Biochim. Biophys. Acta Gen. Subj. 1983, 757, 332–341. [Google Scholar] [CrossRef]
- Huang, T.F.; Holt, J.C.; Lukasiewicz, H.; Niewiarowski, S. Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J. Biol. Chem. 1987, 262, 16157–16163. [Google Scholar] [PubMed]
- Huang, T.F.; Holt, J.C.; Kirby, E.P.; Niewiarowski, S. Trigramin: Primary structure and its inhibition of von willebrand factor binding to glycoprotein IIb/IIIa complex on human platelets. Biochemistry 1989, 28, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, D.A.; Almeida, M.C.; Barros, C.C.; Sanchez, E.F.; Pesquero, P.R.; Lang, E.A.S.; Samaan, M.; Araujo, R.C.; Pesquero, J.B.; Pesquero, J.L. Leucurogin, a new recombinant disintegrin cloned from Bothrops leucurus (white-tailed-jararaca) with potent activity upon platelet aggregation and tumor growth. Toxicon 2011, 58, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Nakada, M.T.; Arnold, C.; Shieh, K.Y.; Markland, F.S., Jr. Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits angiogenesis. Angiogenesis 1999, 3, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; Mousa, S.A.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: A potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003, 63, 2020–2023. [Google Scholar] [PubMed]
- Wang, J.-H.; Wu, Y.; Ren, F.; Lü, L.; Zhao, B.-C. Cloning and characterization of adinbitor, a novel disintegrin from the snake venom of Agkistrodon halys brevicaudus stejneger. Acta Biochim. Biophys. Sin. 2004, 36, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, F.; Wu, Y.; Tian, X.; Wu, Y.; Zhao, B. Cloning, expression and some biological functions of adinbitor, a disintegrin from Agkistrodon halys brevicaudus stejneger. Chin. J. Biochem. Mol. Biol. 2004, 20, 745–749. [Google Scholar]
- Kang, I.-C.; Chung, K.-H.; Lee, S.-J.; Yun, Y.; Moon, H.-M.; Kim, D.-S. Purification and molecular cloning of a platelet aggregation inhibitor from the snake (Agkistrodon halys brevicaudus) venom. Thromb. Res. 1998, 91, 65–73. [Google Scholar] [CrossRef]
- Suhr, S.-M.; Kim, D.-S. Identification of the snake venom substance that induces apoptosis. Biochem. Biophys. Res. Commun. 1996, 224, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Suhr, S.-M.; Kim, D.-S. Comparison of the apoptotic pathways induced by l-amino acid oxidase and hydrogen peroxide. J. Biochem. 1999, 125, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Teng, M.; Niu, L.; Wang, Y.; Liu, Q.; Huang, Q.; Hao, Q.; Dong, Y.; Liu, P. Purification, partial characterization, crystallization and structural determination of AHP-LAAO, a novel l-amino-acid oxidase with cell apoptosis-inducing activity from Agkistrodon halys pallas venom. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Samel, M.; Vija, H.; Rönnholm, G.; Siigur, J.; Kalkkinen, N.; Siigur, E. Isolation and characterization of an apoptotic and platelet aggregation inhibiting l-amino acid oxidase from Vipera berus berus (common viper) venom. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Thangam, R.; Gunasekaran, P.; Kaveri, K.; Sridevi, G.; Sundarraj, S.; Paulpandi, M.; Kannan, S. A novel disintegrin protein from Naja naja venom induces cytotoxicity and apoptosis in human cancer cell lines in vitro. Process Biochem. 2012, 47, 1243–1249. [Google Scholar] [CrossRef]
- Shinako, M.; Hiroshi, H.; Satohiko, A. Two vascular apoptosis-inducing proteins from snake venom are members of the metalloprotease/disintegrin family. Eur. J. Biochem. 1998, 253, 36–41. [Google Scholar] [Green Version]
- Masuda, S.; Araki, S.; Yamamoto, T.; Kaji, K.; Hayashi, H. Purification of a vascular apoptosis-inducing factor from hemorrhagic snake venom. Biochem. Biophys. Res. Commun. 1997, 235, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-P.; Lu, X.-Y.; Wang, X.-F.; Xu, J. Isolation and characterization of a novel P-II class snake venom metalloproteinase from Trimeresurus stejnegeri. Toxicon 2007, 49, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Moreno-Murciano, M.P.; Monleón, D.; Calvete, J.J.; Celda, B.; Marcinkiewicz, C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtusa. Protein Sci. 2003, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.-C.; Lee, Y.-D.; Kim, D.-S. A novel disintegrin salmosin inhibits tumor angiogenesis. Cancer Res. 1999, 59, 3754–3760. [Google Scholar] [PubMed]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Ishida, T.; Yamamoto, T.; Kaji, K.; Hayashi, H. Induction of apoptosis by hemorrhagic snake venom in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1993, 190, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Alsarraj, M.A.; Gasanov, N.E.; Rael, E.D. Cobra venom cytotoxin free of phospholipase A2 and its effect on model membranes and T leukemia cells. J. Membr. Biol. 1997, 155, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Yang, H.L.; Liu, C.Z. Inhibitory effects of immunotargeting of Chinese cobra cytotoxin and iodine-131 against nasopharyngeal carcinoma cells in vitro. J. South. Med. Univ. 2008, 28, 1235–1236. (In Chinese) [Google Scholar]
- Al-Sadoon, M.K.; Abdel-Maksoud, M.A.; Rabah, D.M.; Badr, G. Induction of apoptosis and growth arrest in human breast carcinoma cells by a snake (Walterinnesia aegyptia) venom combined with silica nanoparticles: Crosstalk between Bcl2 and caspase 3. Cell. Physiol. Biochem. 2012, 30, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadoon, M.K.; Rabah, D.M.; Badr, G. Enhanced anticancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: Molecular targets for cell cycle arrest and apoptosis induction. Cell. Immunol. 2013, 284, 129–138. [Google Scholar] [CrossRef] [PubMed]
| Target/Mechanism | Protein Names | Compounds | Snakes | Reference |
|---|---|---|---|---|
| antiangiogenesis | Leucurogin | disintegrin | Bothrops leucurus | [39] |
| Contortrostatin | disintegrin | Agkistrodon contortrix contortrix | [40] | |
| Obtustatin | disintegrin | Vipera lebetina obtusa | [41] | |
| Adinbitor | disintegrin | A. halys brevicaudus stejneger | [42,43] | |
| Salmosin | disintegrin | A. halys brevicaudus | [44] | |
| apoptosis induction | LAAO | LAAO | A. halys | [45,46] |
| AHP-LAAO | LAAO | A. halys pallas | [47] | |
| LAAO | LAAO | V. berus berus | [48] | |
| disintegrin | disintegrin | Naja naja | [49] | |
| VAP and VAP2 | metalloprotease/disintegrin | Crotalus atrox | [50,51] | |
| stejnitin | SVMP | Trimeresurus stejnegeri | [52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346. https://doi.org/10.3390/toxins10090346
Li L, Huang J, Lin Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins. 2018; 10(9):346. https://doi.org/10.3390/toxins10090346
Chicago/Turabian StyleLi, Li, Jianzhong Huang, and Yao Lin. 2018. "Snake Venoms in Cancer Therapy: Past, Present and Future" Toxins 10, no. 9: 346. https://doi.org/10.3390/toxins10090346





