Effective Adsorption of Patulin from Apple Juice by Using Non-Cytotoxic Heat-Inactivated Cells and Spores of Alicyclobacillus Strains
Abstract
:1. Introduction
2. Results
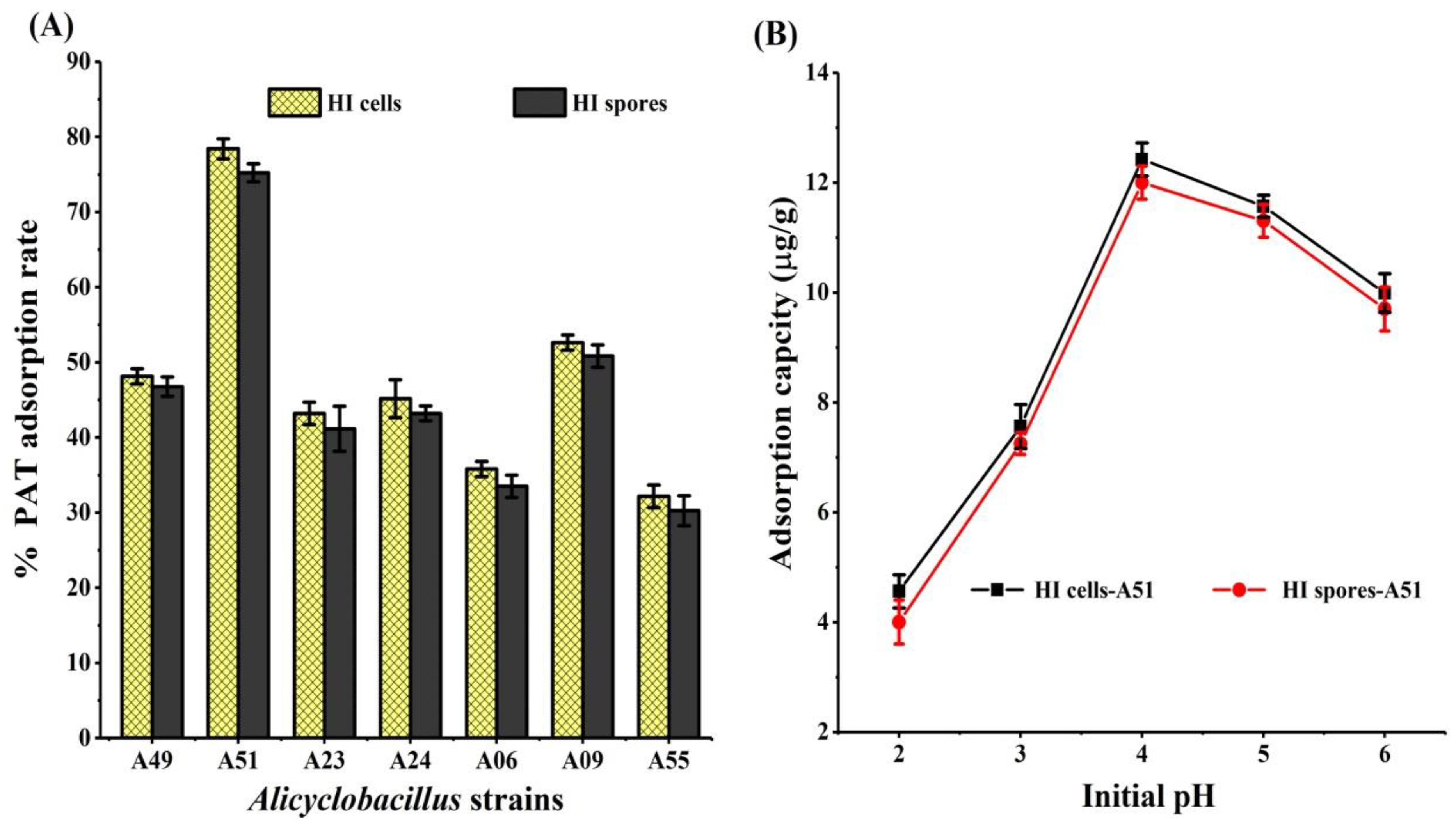
2.1. Adsorption of PAT by HI Cells and Spores of Seven Alicyclobacillus Strains
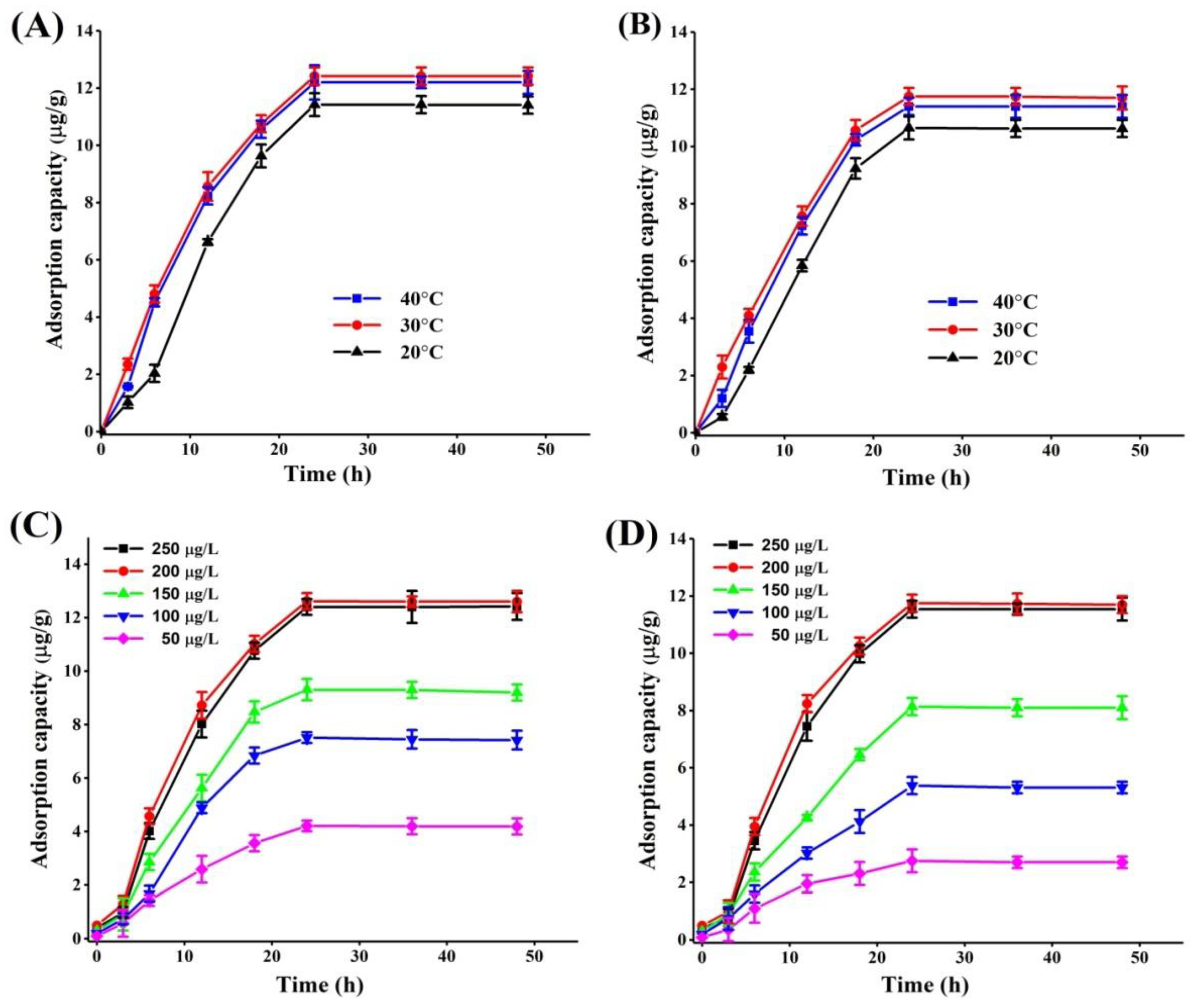
2.2. Effect of Incubation Conditions on PAT Adsorption
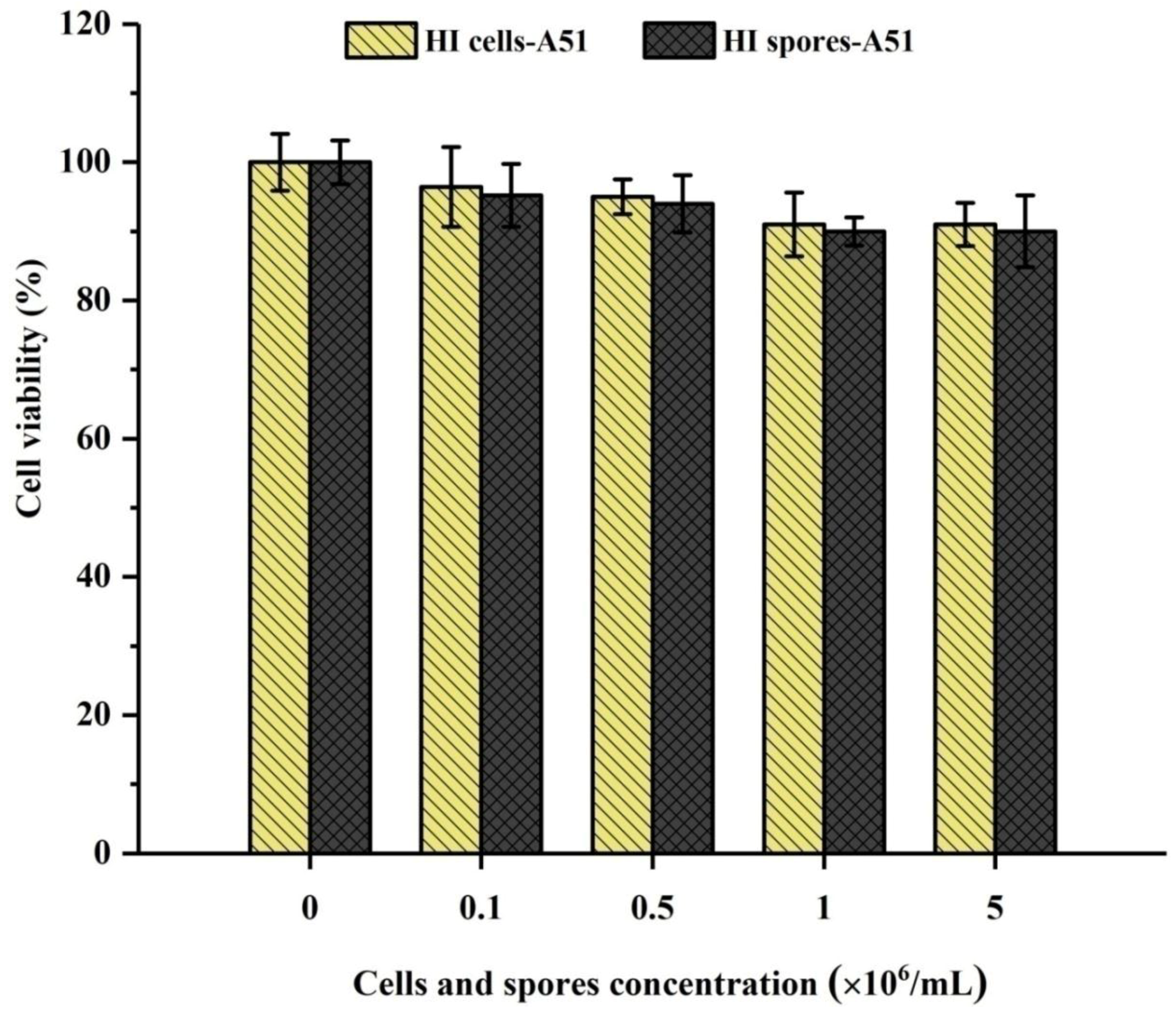
2.3. Cytotoxicity Test
2.4. FTIR Analysis
2.5. Kinetic and Equilibrium Isotherm Modeling
2.5.1. PAT Adsorption Kinetics
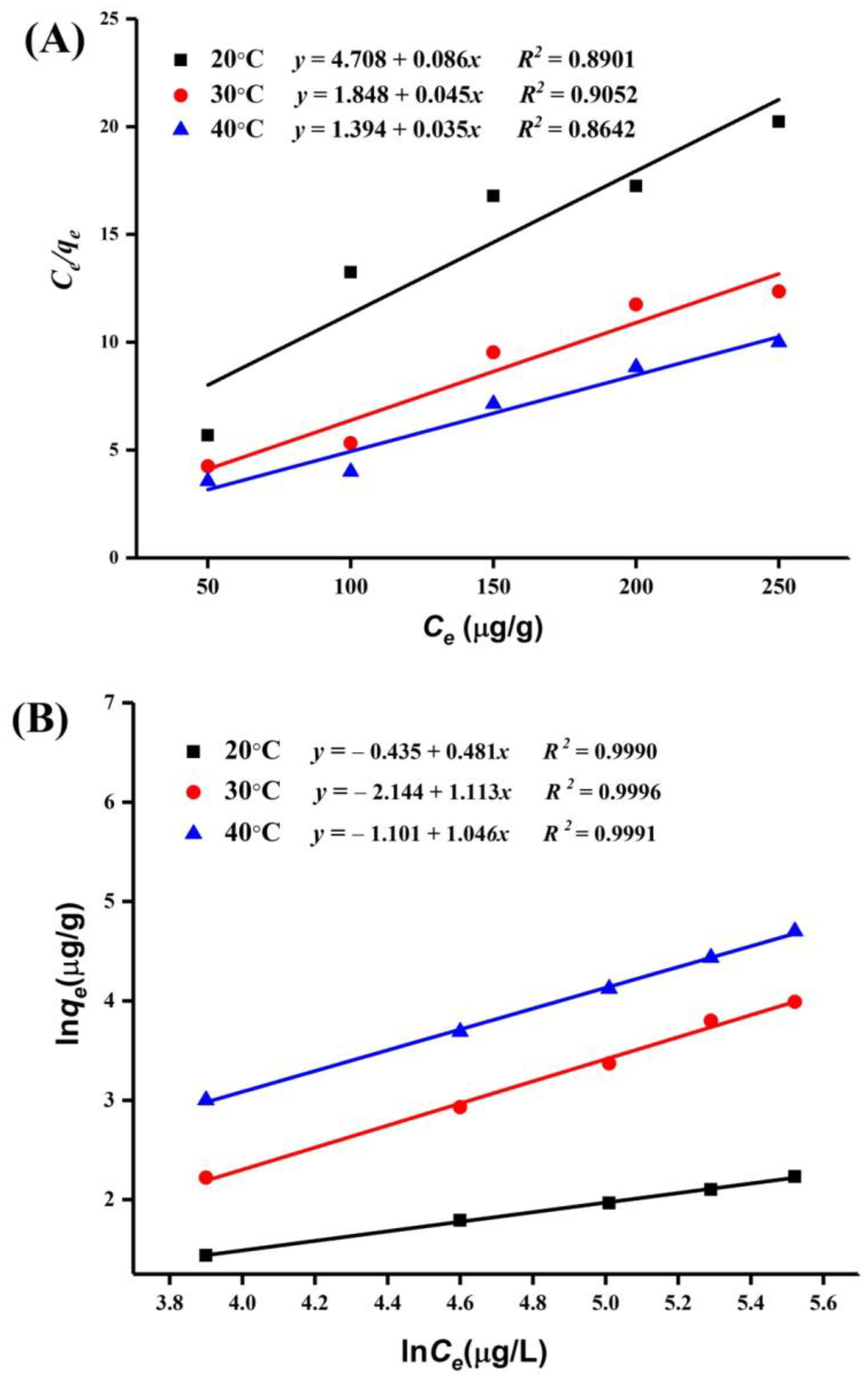
2.5.2. PAT Adsorption Isotherms
2.6. Thermodynamics Parameters
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.1.1. Chemicals and Solvents
5.1.2. Bacterial Strains
5.1.3. Apple Juice
5.2. Preparation of HI Alicyclobacillus Cells and Spores
5.3. Batch Adsorption Experiments
5.4. Quality Parameters of Apple Juice
5.5. In Vitro Cytotoxicity Evaluation
5.6. Characterization
5.7. Sample Extraction and Quantification of PAT
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhu, R.; Feussner, K.; Wu, T.; Yan, F.; Karlovsky, P.; Zheng, X. Detoxification of mycotoxin patulin by the yeast Rhodosporidium paludigenum. Food Chem. 2015, 179, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Kopka, S.; Kaeding, J. Stability of patulin in a juice-like aqueous model system in the presence of ascorbic acid. Food Chem. 2007, 100, 192–197. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Spadaro, D.; Lore, A.; Gullino, M.L.; Garibaldi, A. Potential of patulin production by Penicillium expansum strains on various fruits. Mycotoxin Res. 2010, 26, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Hoeltz, M.; Dottori, H.A.; Noll, I.B. Patulin accumulation in apples during storage by Penicillium expansum and Penicillium griseofulvum strains. Braz. J. Microbiol. 2011, 42, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Frati, S.; Garibaldi, A.; Gullino, M.L.; Berolini, P. Efficacy of different biocontrol agents on the accumulation of patulin by Penicillium expansum in stored apples. In Novel Approaches for the Control of Postharvest Diseases and Disord. Proceedings of the International Congress, Bologna, Italy, 3–5 May 2007; Berolini, P., Ed.; CRIOF, University of Bologna: Bologna, Italy, 2007; pp. 55–59. [Google Scholar]
- World Health Organization. Evaluation of certain food additives and contaminants. Tech. Rep. Ser. 1995, 859, 36–38. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Mansilla, M.L.; Tadeo, J.L. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J. Adv. Res. 2010, 1, 113–122. [Google Scholar] [CrossRef]
- Hao, H.; Zhou, T.; Koutchma, T.; Wu, F.; Warriner, K. High hydrostatic pressure assisted degradation of patulin in fruit and vegetable juice blends. Food Control 2016, 62, 237–242. [Google Scholar] [CrossRef]
- Gonzalez-Osnaya, L.; Soriano, J.M.; Molto, J.C.; Manes, J. Exposure to patulin from consumption of apple-based products. Food Addit. Contam. 2007, 24, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Topcu, A.; Bulat, T.; Wishah, R.; Boyacı, I.H. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010, 139, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Hatab, S.; Yue, T.; Osama, M. Reduction of patulin in aqueous solution by lactic acid bacteria. J. Food Sci. 2012, 77, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Hatab, S.; Yue, T.; Mohamad, O. Removal of patulin from apple juice using inactivated lactic acid bacteria. J. Appl. Microbiol. 2012, 112, 892–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef]
- Fuchs, S.; Sontag, G.; Stidl, R.; Ehrlich, V.; Kundi, M.; Knasmuller, S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008, 46, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yue, T.; Yuan, Y.; Wang, Z.; Ye, M.; Cai, R. A new insight into the adsorption mechanism of patulin by the heat-inactive lactic acid bacteria cells. Food Control 2015, 50, 104–110. [Google Scholar] [CrossRef]
- Ricelli, A.; Baruzzi, F.; Solfrizzo, M.; Morea, M.; Fanizzi, F. Biotransformation of patulin by Gluconobacter oxydans. Appl. Environ. Microbiol. 2007, 73, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Dong, Q.; Guo, C.; Worobo, R.W. Reducing patulin contamination in apple juice by using inactive yeast. J. Food Prot. 2011, 74, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yue, T.; Hatab, S.; Yuan, Y. Ability of inactivated yeast powder to adsorb patulin from apple juice. J. Food Prot. 2012, 75, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, X.; Hatab, S.; Wang, Z.; Wang, Y.; Luo, Y.; Yue, T. Patulin reduction in apple juice by inactivated Alicyclobacillus spp. Lett. Appl. Microbiol. 2014, 59, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Ekebafe, L.O.; Ogbeifun, D.E.; Okieimen, F.E. Equilibrium, kinetic and thermodynamic studies of lead (II) sorption on hydrolyzed starch graft copolymers. J. Polym. Environ. 2018, 26, 807–818. [Google Scholar] [CrossRef]
- Ringot, D.; Lerzy, B.; Bonhoure, J.P.; Auclair, E.; Oriol, E.; Larondell, Y. Effect of temperature on in vitro ochratoxin A biosorption onto yeast cell wall derivatives. Process Biochem. 2005, 40, 3008–3016. [Google Scholar] [CrossRef]
- Yue, T.; Guo, C.; Yuan, Y.; Wang, Z.; Luo, Y.; Wang, L. Adsorptive removal of patulin from apple juice using Ca-alginate-activated carbon beads. J. Food Sci. 2013, 78, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachlouei, B.; Ahmadi-Zenouz, A.; Assadi, Y.; Hesari, J. Reduction of patulin content in apple juice concentrate using activated carbon and its effects on several chemical constituents. J. Food Agric. Environ. 2007, 5, 12–16. [Google Scholar]
- Peng, X.; Liu, B.; Chen, W.; Li, X.; Wang, Q.; Meng, X.; Wang, D. Effective biosorption of patulin from apple juice by cross-linked xanthated chitosan resin. Food Control 2016, 63, 140–146. [Google Scholar] [CrossRef]
- Tang, M.; Li, X.; Gao, C.; Li, X.; Qiu, H. Adsorption performance of CuFe2O4/rGOnano composites towards organic dye. Mater. Chem. Phys. 2016, 185, 114–121. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, H.; Guo, C.; Zheng, J.; Yue, T.; Yuan, Y. One-step preparation of nano-Fe3O4 modified inactivated yeast for the adsorption of patulin. Food Control 2018, 86, 310–318. [Google Scholar] [CrossRef]
- Schiewer, S.; Patil, S.B. Pectin rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, Z.; Yue, T. Synthesis and characterization of nontoxic chitosan-coated Fe3O4 particles for patulin adsorption in a juice-pH simulation aqueous. Food Chem. 2017, 221, 317–323. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Torlak, E. Inactivation of Alicyclobacillus acidoterrestris spores in aqueous suspension and on apples by neutral electrolyzed water. Int. J. Food Microbiol. 2014, 185, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Investigation of the cytotoxicity of food-grade nanoemulsions in Caco-2 cell monolayers and HepG2 cells. Food Chem. 2013, 141, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhuang, H.; Zhang, T.; Liu, J. Patulin content in apple products marketed in Northeast China. Food Control 2010, 21, 1488–1491. [Google Scholar] [CrossRef]
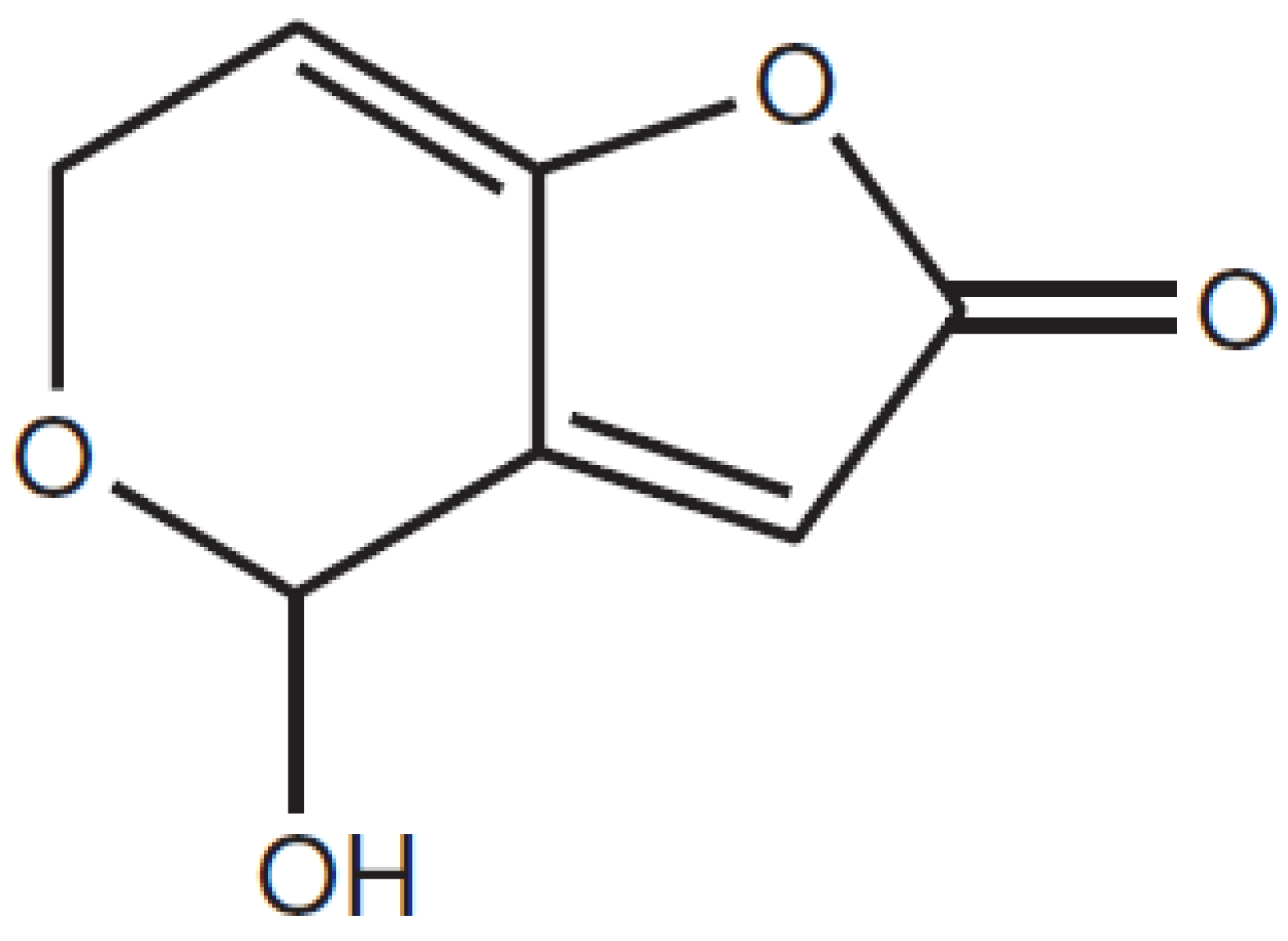


| Strains or Matrix | Quality Parameters | ||||
|---|---|---|---|---|---|
| °Brix | Total Sugar (g/100 mL) | Titratable Acidity (g/100 mL) | Color Value (%) | Clarity (%) | |
| CK1* | 12.58 ± 0.37 | 11.43 ± 0.04 | 0.34 ± 0.03 | 86.43 ± 1.67 | 95.43 ± 0.37 |
| CK2* (Control) | 12.75 ± 0.52 | 10.61 ± 0.06 | 0.33 ± 0.01 | 80.51 ± 2.74 | 93.17 ± 0.46 |
| HI cells-A49 | 12.43 ± 0.56 | 10.79 ± 0.15 | 0.33 ± 0.03 | 81.57 ± 1.53 | 93.73 ± 1.73 |
| HI spores-49 | 12.96 ± 0.26 | 11.27 ± 0.18 | 0.35 ± 0.05 | 80.32 ± 1.24 | 92.03 ± 2.34 |
| HI cells-A51 | 12.60 ± 0.38 | 11.00 ± 0.03 | 0.33 ± 0.02 | 85.10 ± 3.48 | 95.73 ± 167 |
| HI spores-A51 | 12.64 ± 0.46 | 10.86 ± 0.06 | 0.34 ± 0.08 | 85.90 ± 3.17 | 94.73 ± 0.62 |
| HI cells-A23 | 12.85 ± 0.50 | 11.59 ± 0.09 | 0.34 ± 0.06 | 81.62 ± 2.21 | 96.07 ± 0.49 |
| HI spores-A23 | 12.78 ± 0.50 | 10.64 ± 0.19 | 0.36 ± 0.02 | 81.34 ± 2.63 | 94.93 ± 0.16 |
| HI cells-A24 | 12.72 ± 0.25 | 11.62 ± 0.08 | 0.35 ± 0.06 | 82.38 ± 1.81 | 95.79 ± 1.90 |
| HI spores-A24 | 13.00 ± 0.12 | 11.75 ± 0.36 | 0.34 ± 0.02 | 88.42 ± 0.49 | 96.36 ± 0.86 |
| HI cells-A06 | 13.00 ± 0.16 | 10.84 ± 0.38 | 0.33 ± 0.03 | 30.29 ± 1.59 | 53.97 ± 0.48 |
| HI spores-A06 | 12.92 ± 0.58 | 11.72 ± 0.26 | 0.35 ± 0.01 | 36.44 ± 1.39 | 50.84 ± 1.09 |
| HI cells-A09 | 12.86 ± 0.43 | 10.85 ± 0.06 | 0.36 ± 0.03 | 81.38 ± 2.47 | 93.16 ± 0.26 |
| HI spores-A09 | 13.00 ± 0.53 | 10.79 ± 0.13 | 0.33 ± 0.03 | 81.89 ± 2.76 | 93.07 ± 0.37 |
| HI cells-A55 | 12.76 ± 0.59 | 11.82 ± 0.06 | 0.34 ± 0.02 | 75.29 ± 2.15 | 95.97 ± 0.35 |
| HI spores-A55 | 12.83 ± 0.25 | 10.56 ± 0.09 | 0.33 ± 0.05 | 80.19 ± 0.78 | 92.29 ± 1.64 |
| Adsorbent | - | Pseudo-First Order Kinetic Model | Pseudo-Second Order Kinetic Model | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | ||||||||
| HI cells-A51 | Temperature (°C) | |||||||
| 20 | 11.426 | 0.052 | 13.144 | 0.950 | 0.0029 | 23.951 | 0.753 | |
| 30 | 12.621 | 0.069 | 16.995 | 0.999 | 0.0048 | 24.541 | 0.892 | |
| 40 | 12.203 | 0.062 | 16.014 | 0.982 | 0.0037 | 24.236 | 0.930 | |
| Initial PAT concn. (μg/L) | ||||||||
| 50 | 4.211 | 0.098 | 6.423 | 0.918 | 0.019 | 5.681 | 0.901 | |
| 100 | 7.512 | 0.075 | 9.510 | 0.980 | 0.0073 | 11.415 | 0.830 | |
| 150 | 9.309 | 0.089 | 11.142 | 0.943 | 0.0063 | 12.725 | 0.911 | |
| 200 | 12.621 | 0.069 | 16.995 | 0.999 | 0.0048 | 24.541 | 0.892 | |
| 250 | 12.403 | 0.060 | 14.817 | 0.962 | 0.0025 | 21.426 | 0.745 | |
| HI spores-A51 | Temperature (°C) | |||||||
| 20 | 10.652 | 0.051 | 13.144 | 0.924 | 0.0022 | 16.304 | 0.628 | |
| 30 | 11.751 | 0.063 | 14.971 | 0.998 | 0.0038 | 23.985 | 0.873 | |
| 40 | 11.402 | 0.060 | 14.325 | 0.959 | 0.0025 | 23.457 | 0.901 | |
| Initial PAT concn. (μg/L) | ||||||||
| 50 | 2.753 | 0.099 | 5.315 | 0.901 | 0.0140 | 5.025 | 0.814 | |
| 100 | 5.378 | 0.061 | 7.361 | 0.968 | 0.0049 | 10.950 | 0.733 | |
| 150 | 8.140 | 0.074 | 10.78 | 0.953 | 0.0051 | 11.862 | 0.913 | |
| 200 | 11.751 | 0.057 | 14.971 | 0.998 | 0.0038 | 23.985 | 0.873 | |
| 250 | 11.541 | 0.051 | 13.781 | 0.925 | 0.0022 | 21.056 | 0.719 | |
| Adsorbents | Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 1/n | ||||||
| 20 | 11.759 | 1.214 | 0.9035 | 0.147 | 0.254 | 0.9991 | |
| HI cells-A51 | 30 | 12.755 | 1.356 | 0.9462 | 0.259 | 0.398 | 0.9998 |
| 40 | 12.207 | 1.213 | 0.9119 | 0.201 | 0.303 | 0.9992 | |
| 20 | 10.956 | 1.031 | 0.8901 | 0.110 | 0.241 | 0.9990 | |
| HI spores-A51 | 30 | 11.823 | 1.244 | 0.9052 | 0.137 | 0.364 | 0.9996 |
| 40 | 11.710 | 1.185 | 0.8642 | 0.131 | 0.301 | 0.9991 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, M.; Mehmood, S.; Niu, C.; Yuan, Y.; Yue, T. Effective Adsorption of Patulin from Apple Juice by Using Non-Cytotoxic Heat-Inactivated Cells and Spores of Alicyclobacillus Strains. Toxins 2018, 10, 344. https://doi.org/10.3390/toxins10090344
Sajid M, Mehmood S, Niu C, Yuan Y, Yue T. Effective Adsorption of Patulin from Apple Juice by Using Non-Cytotoxic Heat-Inactivated Cells and Spores of Alicyclobacillus Strains. Toxins. 2018; 10(9):344. https://doi.org/10.3390/toxins10090344
Chicago/Turabian StyleSajid, Marina, Sajid Mehmood, Chen Niu, Yahong Yuan, and Tianli Yue. 2018. "Effective Adsorption of Patulin from Apple Juice by Using Non-Cytotoxic Heat-Inactivated Cells and Spores of Alicyclobacillus Strains" Toxins 10, no. 9: 344. https://doi.org/10.3390/toxins10090344





