Butylbenzene and tert-Butylbenzene—Sorption on Sand Particles and Biodegradation in the Presence of Plant Natural Surfactants
Abstract
:1. Introduction
2. Results and Discussion
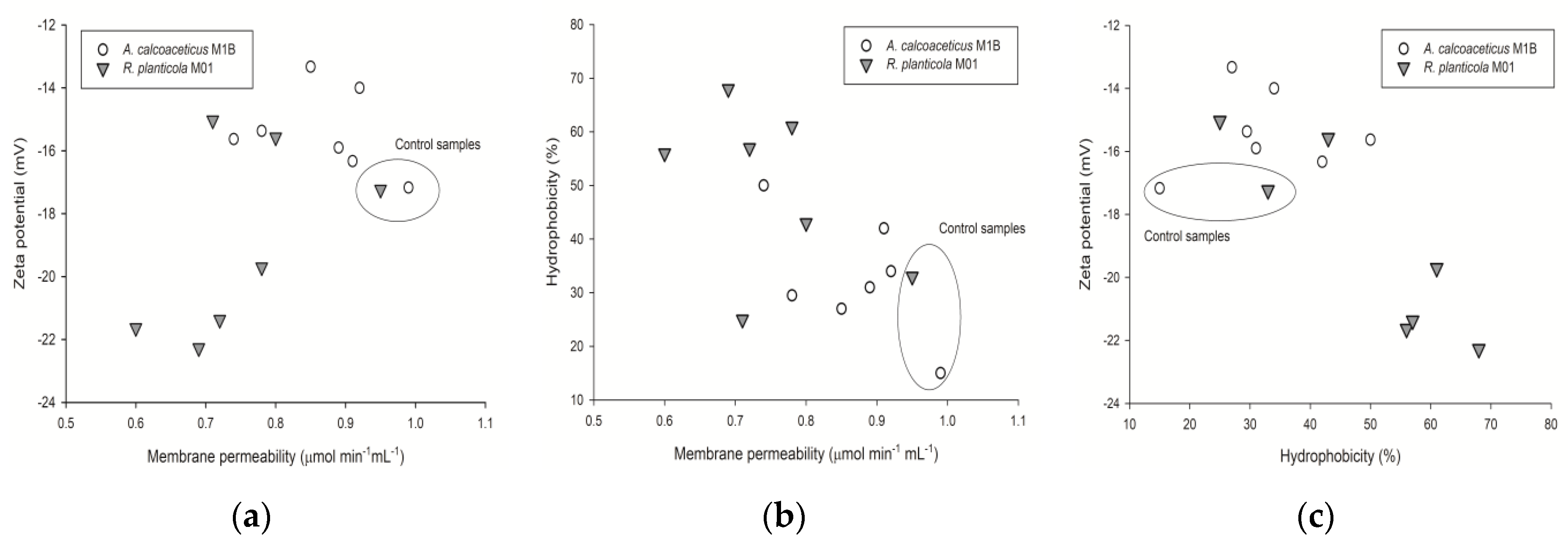
2.1. Microbes Surface Properties
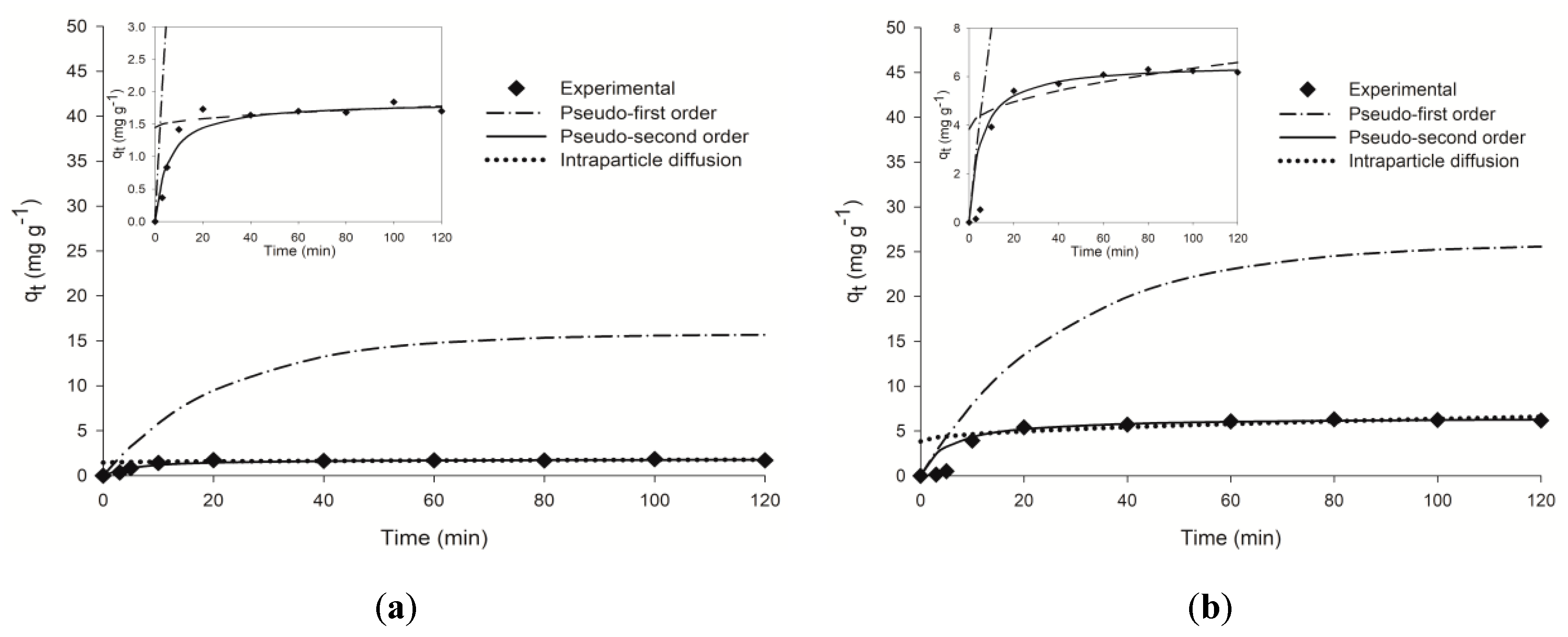
2.2. Sorption Kinetics
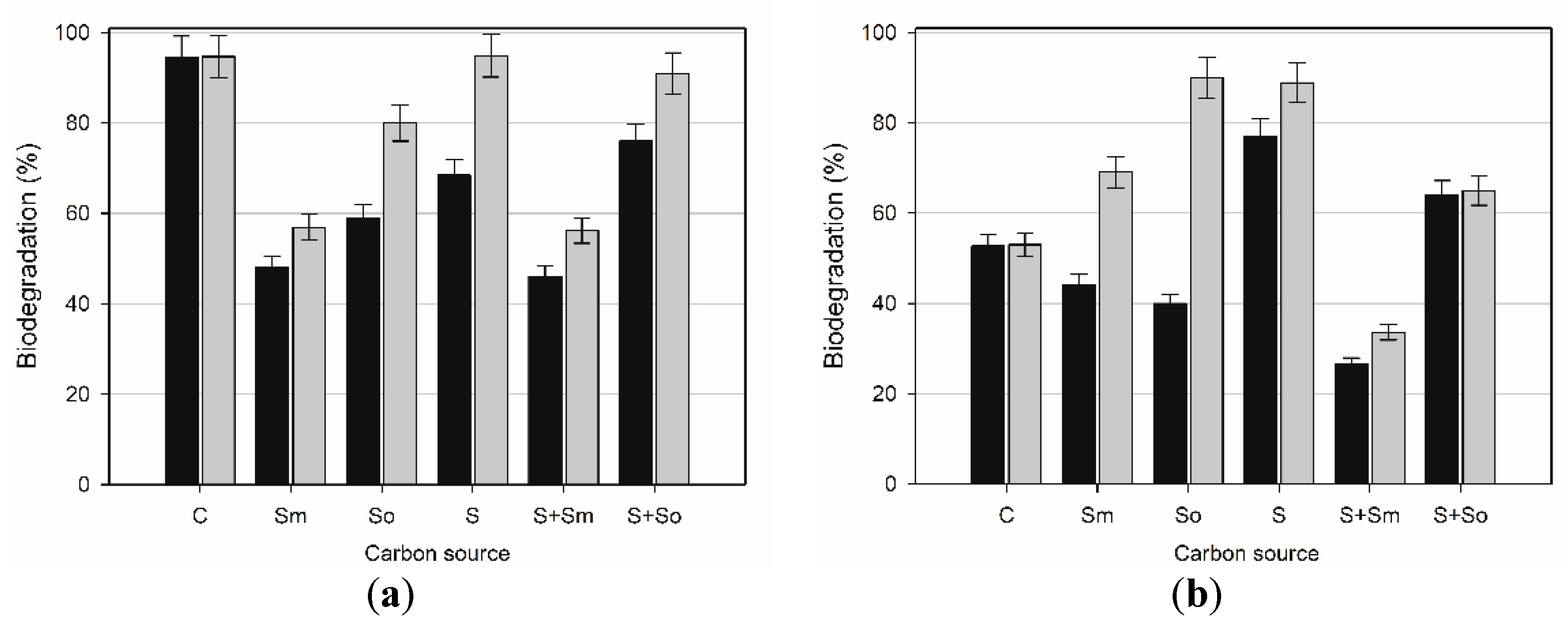
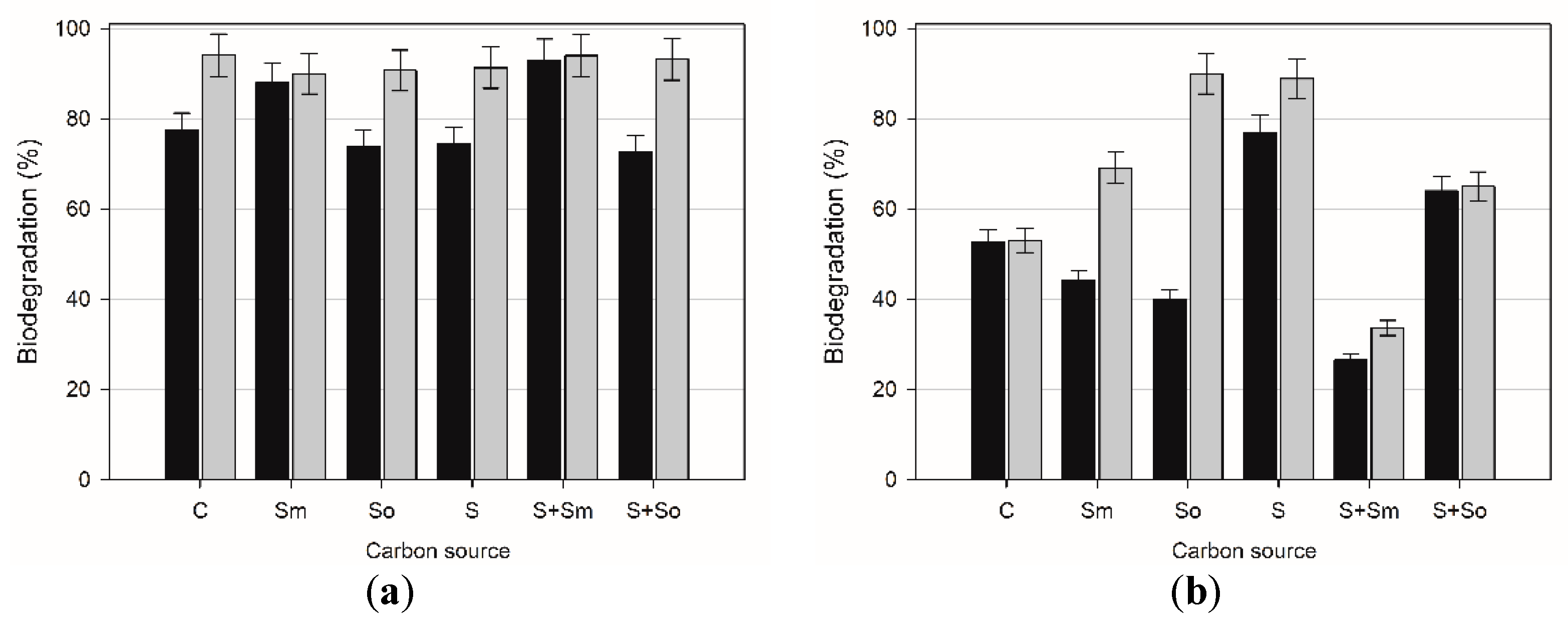
2.3. Hydrocarbons Biodegradation
3. Conclusions
4. Materials and Methods
4.1. Strains, Surfactants and Chemicals
4.2. Microbes Surface Properties Measurement: Cell Surface Hydrophobicity, Zeta Potential and Membrane Permeability
4.3. Adsorption Kinetics Modeling
4.4. Hydrocarbons Biodegradation
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Boopathy, R.; Shields, S.; Nunna, S. Biodegradation of crude oil from the BP Oil spill in the marsh sediments of Southeast Louisiana, USA. Appl. Biochem. Biotechnol. 2012, 167, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.W. Avoidance of oil contaminated sediments by estuarine fishes. Mar. Ecol. Prog. Ser. 2017, 576, 125–134. [Google Scholar] [CrossRef]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Venny, G.S.; Ng, H.K. Current status and prospects of Fenton oxidation for the decontamination of persistent organic pollutants (POPs) in soils. Chem. Eng. J. 2012, 213, 295–317. [Google Scholar] [CrossRef]
- Gomes, H.I.; Dias-Ferreira, C.; Ottosen, L.M.; Ribeiro, A.B. Electroremediation of PCB contaminated soil combined with iron nanoparticles: Effect of the soil type. Chemosphere 2015, 131, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A.; Razzak, S.; Zhu, J. Bioaugmentation: An emerging strategy of industrial wastewater treatment for reuse and discharge. Int. J. Environ. Res. Public Health 2016, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Sharma, R.; Singh, K.; Sharma, A. Application of bioremediation technology in the environment contaminated with petroleum hydrocarbon. Ann. Microbiol. 2013, 63, 417–431. [Google Scholar] [CrossRef]
- McGenity, T.J. Hydrocarbon biodegradation in intertidal wetland sediments. Curr. Opin. Biotechnol. 2014, 27, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Jasmine, J.; Mukherji, S. Practical considerations and challenges involved in surfactant enhanced bioremediation of oil. BioMed Res. Int. 2013, 2013, 328608. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Anselmo, A.M.; Suzuki, S.; Mendo, S. Tributyltin (TBT): A Review on microbial resistance and degradation. Crit. Rev. Environ. Sci. Technol. 2015, 45, 970–1006. [Google Scholar] [CrossRef]
- Salleh, A.B.; Ghazali, F.M.; Rahman, R.N.Z.A.; Basri, M. Bioremediation of petroleum hydrocarbon pollution. Indian J. Biotechnol. 2003, 2, 411–425. [Google Scholar]
- Krell, T.; Lacal, J.; Reyes-Darias, J.A.; Jimenez-Sanchez, C.; Sungthong, R.; Ortega-Calvo, J.J. Bioavailability of pollutants and chemotaxis. Curr. Opin. Biotechnol. 2013, 24, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errington, I.; King, C.K.; Wilkins, D.; Spedding, T.; Hose, G.C. Ecosystem effects and the management of petroleum-contaminated soils on subantarctic islands. Chemosphere 2018, 194, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Beulke, S.; Brown, C.D. Factors influencing degradation of pesticides in soil. J. Agric. Food Chem. 2007, 55, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Ciffroy, P. Modelling the fate of chemicals in soils. In Modelling the Fate of Chemicals in the Environment and the Human Body; Ciffroy, P., Tediosi, A., Capri, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 127–147. ISBN 978-3-319-59502-3. [Google Scholar]
- Pan, T.; Miao, T.; Yu, Q. Predictive model for propagation of beneficial microbes in variably saturated soils. Environ. Eng. Sci. 2017, 34, 385–393. [Google Scholar] [CrossRef]
- Calvo, O.; Julio, J.; Fernández, G.; Victoria, C. Role of natural surfactants in bioremediation and bioavailability of PAH. Biosaia 2016, 4, 10877. [Google Scholar]
- Bueno-Montes, M.; Springael, D.; Ortega-Calvo, J.-J. Effect of a nonionic surfactant on biodegradation of slowly desorbing PAHs in contaminated soils. Environ. Sci. Technol. 2011, 45, 3019–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Zhu, L. Effect of surfactant-induced cell surface modifications on electron transport system and catechol 1,2-dioxygenase activities and phenanthrene biodegradation by Citrobacter sp. SA01. Bioresour. Technol. 2012, 123, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Smułek, W.; Zdarta, A.; Guzik, U.; Dudzińska-Bajorek, B.; Kaczorek, E. Rahnella sp. strain EK12: Cell surface properties and diesel oil biodegradation after long-term contact with natural surfactants and diesel oil. Microbiol. Res. 2015, 176, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Khire, J.M. Bacterial biosurfactants, and their role in microbial enhanced oil recovery (MEOR). In Biosurfactants; Ramkrishna, S., Ed.; Springer: New York, NY, USA, 2010; pp. 146–157. [Google Scholar]
- Thaniyavarn, J.; Roongsawang, N.; Kameyama, T.; Haruki, M.; Imanaka, T.; Morikawa, M.; Kanaya, S. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci. Biotechnol. Biochem. 2003, 67, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Vasileva-Tonkova, E.; Sotirova, A.; Galabova, D. The effect of rhamnolipid biosurfactant produced by Pseudomonas fluorescens on model bacterial strains and isolates from industrial wastewater. Curr. Microbiol. 2011, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Saharan, B.S.; Sahu, R.K.; Sharma, D. A review on biosurfactants: Fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2011, 2011, 1–14. [Google Scholar]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Yang, C.-H.; Chang, M.-S.; Ciou, Y.-P.; Huang, Y.-C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, Y.J.; Nam, K. Enhancement of aerobic biodegradation in an oxygen-limiting environment using a saponin-based microbubble suspension. Environ. Pollut. 2009, 157, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Jesionowski, T.; Giec, A.; Olszanowski, A. Cell surface properties of Pseudomonas stutzeri in the process of diesel oil biodegradation. Biotechnol. Lett. 2012, 34, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kaminaga, H.; Navarro, R.R.; Iimura, Y. Application of aqueous saponin on the remediation of polycyclic aromatic hydrocarbons-contaminated soil. J. Environ. Sci. Heal. Part A 2012, 47, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Smułek, W.; Zdarta, A.; Łuczak, M.; Krawczyk, P.; Jesionowski, T.; Kaczorek, E. Sapindus saponins’ impact on hydrocarbon biodegradation by bacteria strains after short- and long-term contact with pollutant. Colloids Surf. B Biointerfaces 2016, 142, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.; Martinez, N.; Diaz, G. Content of the saponin protodioscin in Brachiaria spp. from the eastern plains of Colombia. Toxins 2017, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-F.; Zeng, G.-M.; Zhong, H.; Yuan, X.-Z.; Jiang, L.; Fu, H.-Y.; Ma, X.; Zhang, J.-C. Effect of saponins on cell surface properties of Penicillium simplicissimum: Performance on adsorption of cadmium(II). Colloids Surf. B Biointerfaces 2011, 86, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, Y.; Zhong, H.; Xiao, R.; Zeng, G.; Liu, Z.; Cheng, M.; Lai, C.; Zhang, C.; Liu, G.; et al. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds. Sci. Total Environ. 2018, 634, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Sałek, K.; Guzik, U.; Jesionowski, T.; Cybulski, Z. Biodegradation of alkyl derivatives of aromatic hydrocarbons and cell surface properties of a strain of Pseudomonas stutzeri. Chemosphere 2013, 90, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zhu, Y.; Cai, X.; Shi, W.; Li, H. Surfactant flushing remediation of o-dichlorobenzene and p-dichlorobenzene contaminated soil. Chemosphere 2017, 185, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Smułek, W.; Zdarta, A.; Sawczuk, A.; Zgoła-Grześkowiak, A. Influence of saponins on the biodegradation of halogenated phenols. Ecotoxicol. Environ. Saf. 2016, 131, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Selvam, A.; Wong, J.W.-C. Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene. Bioresour. Technol. 2011, 102, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Song, R.; Du, G.; Li, H.; Chen, J. Effects of EDTA and Tween60 on biodegradation of n-hexadecane with two strains of Pseudomonas aeruginosa. Biochem. Eng. J. 2007, 36, 66–71. [Google Scholar] [CrossRef]
- Yalçin, E.; Cavuşoğlu, K.; Ozen, E. Hydrocarbon degradation by a new Pseudomonas sp., strain RW-II, with polycationic surfactant to modify the cell hydrophobicity. Environ. Technol. 2011, 33, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and chromium from aqueous solutions using red mud—An aluminium industry waste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Equilibria and capacities for adsorption on carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Bandura, L.; Kołodyńska, D.; Franus, W. Adsorption of BTX from aqueous solutions by Na-P1 zeolite obtained from fly ash. Process Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Moura, C.P.; Vidal, C.B.; Barros, A.L.; Costa, L.S.; Vasconcellos, L.C.G.; Dias, F.S.; Nascimento, R.F. Adsorption of BTX (benzene, toluene, o-xylene, and p-xylene) from aqueous solutions by modified periodic mesoporous organosilica. J. Colloid Interface Sci. 2011, 363, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.N.; da Motta, M.; Benachour, M.; Sales, D.C.S.; Abreu, C.A.M. Evaluation of BTEX and phenol removal from aqueous solution by multi-solute adsorption onto smectite organoclay. J. Hazard. Mater. 2012, 239–240, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Li, L.; Zhang, Q.; Wu, H. Equilibrium, kinetics and thermodynamic studies for sorption of chlorobenzenes on CTMAB modified bentonite and kaolinite. J. Hazard. Mater. 2010, 173, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hackbarth, F.V.; Vilar, V.J.P.; De Souza, G.B.; de Souza, S.M.A.G.U.; de Souza, A.A.U. Benzene, toluene and o-xylene (BTX) removal from aqueous solutions through adsorptive processes. Adsorption 2014, 20, 577–590. [Google Scholar] [CrossRef]
- Aivalioti, M.; Vamvasakis, I.; Gidarakos, E. BTEX and MTBE adsorption onto raw and thermally modified diatomite. J. Hazard. Mater. 2010, 178, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, J.T.; da Conceição, M.; Alvim-Ferraz, M.; Delerue-Matos, M.C.F. Estimation of pollutant partition in sandy soils with different water contents. Environ. Monit. Assess. 2010, 171, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Guo, C.; Liang, X.; Wu, F.; Dang, Z. Nonionic surfactants induced changes in cell characteristics and phenanthrene degradation ability of Sphingomonas sp. GY2B. Ecotoxicol. Environ. Saf. 2016, 129, 210–218. [Google Scholar] [CrossRef] [PubMed]
- White, B.E.; Fenner, C.J.; Smit, M.S.; Harrison, S.T.L. Effect of cell permeability and dehydrogenase expression on octane activation by CYP153A6-based whole cell Escherichia coli catalysts. Microb. Cell Fact. 2017, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Ratledge, C. Catabolism of alkylbenzenes by Pseudomonas sp. NCIB 10643. Appl. Microbiol. Biotechnol. 1989, 32, 68–75. [Google Scholar] [CrossRef]
- Govarthanan, M.; Fuzisawa, S.; Hosogai, T.; Chang, Y.-C. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Pacholak, A.; Zgoła-Grześkowiak, A.; Marczak, Ł.; Jarzębski, M.; Kaczorek, E. Saponaria officinalis L. extract: Surface active properties and impact on environmental bacterial strains. Colloids Surf. B Biointerfaces 2017, 150, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Moszyńska, S.; Olszanowski, A. Modification of cell surface properties of Pseudomonas alcaligenes S22 during hydrocarbon biodegradation. Biodegradation 2011, 22, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, A.; Dudzińska-Bajorek, B.; Nowak, A.; Guzik, U.; Kaczorek, E. Impact of potent bioremediation enhancing plant extracts on Raoultella ornithinolytica properties. Ecotoxicol. Environ. Saf. 2017, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, L.; Li, F. Influences and mechanisms of surfactants on pyrene biodegradation based on interactions of surfactant with a Klebsiella oxytoca strain. Bioresour. Technol. 2013, 142, 454–461. [Google Scholar] [CrossRef] [PubMed]

| Kinetic Model | Equation | Isotherm Model | Equation |
|---|---|---|---|
| Pseudo-first order | Langmuir | ||
| Pseudo-second order | Freundlich | ||
| Intraparticle diffusion | |||
| Kinetic Model | Hydrocarbon | Parameters | Value | R2 |
|---|---|---|---|---|
| Pseudo-first order | BB | kf (min−1) | 0.04606 | 0.924 |
| qe (mg g−1) | 15.7398 | |||
| TB | kf (min−1) | 0.03685 | 0.972 | |
| qe (mg g−1) | 25.8821 | |||
| Pseudo-second order | BB | ks (g mg−1 min−1) | 0.09721 | 0.992 |
| qe (mg g−1) | 1.84502 | |||
| h0 (mg g−1 min−1) | 0.33091 | |||
| TB | ks (g mg−1 min−1) | 0.03009 | 0.998 | |
| qe (mg g−1) | 6.53595 | |||
| h0 (mg g−1 min−1) | 1.28535 | |||
| Intraparticle diffusion | BB | kid (mg g−1 min−0.5) | 0.030 | 0.460 |
| TB | kid (mg g−1 min−0.5) | 0.251 | 0.741 |
| Isotherm Model | Hydrocarbon | Parameters | Value | R2 |
|---|---|---|---|---|
| Langmuir | BB | a (mL mg−1) | 2.15054 | 0.916 |
| b (mL g−1) | 2.59777 | |||
| TB | a (mL mg−1) | 0.59595 | 0.982 | |
| b (mL g−1) | 5.21118 | |||
| Freundlich | BB | KF (mg g−1) | 0.478 | 0.987 |
| n | 1.20048 | |||
| TB | KF (mg g−1) | 0.074 | 0.997 | |
| n | 1.27064 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdarta, A.; Pacholak, A.; Galikowska, M.; Smułek, W.; Kaczorek, E. Butylbenzene and tert-Butylbenzene—Sorption on Sand Particles and Biodegradation in the Presence of Plant Natural Surfactants. Toxins 2018, 10, 338. https://doi.org/10.3390/toxins10090338
Zdarta A, Pacholak A, Galikowska M, Smułek W, Kaczorek E. Butylbenzene and tert-Butylbenzene—Sorption on Sand Particles and Biodegradation in the Presence of Plant Natural Surfactants. Toxins. 2018; 10(9):338. https://doi.org/10.3390/toxins10090338
Chicago/Turabian StyleZdarta, Agata, Amanda Pacholak, Marta Galikowska, Wojciech Smułek, and Ewa Kaczorek. 2018. "Butylbenzene and tert-Butylbenzene—Sorption on Sand Particles and Biodegradation in the Presence of Plant Natural Surfactants" Toxins 10, no. 9: 338. https://doi.org/10.3390/toxins10090338





