Phoneutria nigriventer Spider Toxin PnTx2-1 (δ-Ctenitoxin-Pn1a) Is a Modulator of Sodium Channel Gating
Abstract
:1. Introduction
2. Results
2.1. Electrophysiological Charaterization
2.1.1. Activity of PnTx2-1 on Mammalian NaV Channels
2.1.2. Activity of PnTx2-1 on Insect NaV-Channel Currents
2.1.3. Activity of PnTx2-6 on NaV1.5-Channel Currents
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Toxin Purification
5.2. Electrophysiology
5.2.1. Heterologous Expression in Xenopus laevis oocytes
5.2.2. Electrophysiological Recordings
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pineda, S.S.; Undheim, E.A.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider venomics: Implications for drug discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.S.; Chaumeil, P.A.; Kunert, A.; Kaas, Q.; Thang, M.W.C.; Le, L.; Nuhn, M.; Herzig, V.; Saez, N.J.; Cristofori-Armstrong, B.; et al. ArachnoServer 3.0: An online resource for automated discovery, analysis and annotation of spider toxins. Bioinformatics 2018, 34, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P. Molecular diversification in spider venoms: A web of combinatorial peptide libraries. Mol. Divers. 2006, 10, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Klint, J.K.; Senff, S.; Rupasinghe, D.B.; Er, S.Y.; Herzig, V.; Nicholson, G.M.; King, G.F. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon 2012, 60, 478–491. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; de Lima, M.E.; Tytgat, J. Phoneutria nigriventer venom: A pharmacological treasure. Toxicon 2018, 151, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Herzig, V.; John Ward, R.; Ferreira dos Santos, W. Intersexual variations in the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer (Keyserling, 1891). Toxicon 2002, 40, 1399–1406. [Google Scholar] [CrossRef]
- Bucaretchi, F.; Mello, S.M.; Vieira, R.J.; Mamoni, R.L.; Blotta, M.H.; Antunes, E.; Hyslop, S. Systemic envenomation caused by the wandering spider Phoneutria nigriventer, with quantification of circulating venom. Clin. Toxicol. 2008, 46, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.R.V.; Paiva, A.L.B.; Guerra-Duarte, C.; Nishiyama, M.Y., Jr.; Mudadu, M.A.; Oliveira, U.; Borges, M.H.; Yates, J.R.; Junqueira-de-Azevedo, I.L. An overview of Phoneutria nigriventer spider venom using combined transcriptomic and proteomic approaches. PLoS ONE 2018, 13, e0200628. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.R.; Cordeiro Mdo, N.; Junor, L.R.; Kelly, P.; Fischer, S.; Reimann, F.; Oliveira, E.B.; Richardson, M. The purification and amino acid sequence of the lethal neurotoxin Tx1 from the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer. FEBS Lett. 1990, 263, 251–253. [Google Scholar] [CrossRef]
- King, G.F.; Gentz, M.C.; Escoubas, P.; Nicholson, G.M. A rational nomenclature for naming peptide toxins from spiders and other venomous animals. Toxicon 2008, 52, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, S.G.; Garcia, M.E.; Valentim, A.C.; Cordeiro, M.N.; Diniz, C.R.; Richardson, M. Purification and amino acid sequence of the insecticidal neurotoxin Tx4(6-1) from the venom of the ‘armed’ spider Phoneutria nigriventer (Keys). Toxicon 1995, 33, 83–93. [Google Scholar] [CrossRef]
- Cordeiro Mdo, N.; Diniz, C.R.; Valentim Ado, C.; von Eickstedt, V.R.; Gilroy, J.; Richardson, M. The purification and amino acid sequences of four Tx2 neurotoxins from the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer (Keys). FEBS Lett. 1992, 310, 153–156. [Google Scholar] [CrossRef]
- Matavel, A.; Fleury, C.; Oliveira, L.C.; Molina, F.; de Lima, M.E.; Cruz, J.S.; Cordeiro, M.N.; Richardson, M.; Ramos, C.H.; Beirao, P.S. Structure and activity analysis of two spider toxins that alter sodium channel inactivation kinetics. Biochemistry 2009, 48, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Matavel, A.; Cruz, J.S.; Penaforte, C.L.; Araujo, D.A.; Kalapothakis, E.; Prado, V.F.; Diniz, C.R.; Cordeiro, M.N.; Beirao, P.S. Electrophysiological characterization and molecular identification of the Phoneutria nigriventer peptide toxin PnTx2-6. FEBS Lett. 2002, 523, 219–223. [Google Scholar] [CrossRef]
- Catterall, W.A.; Cestele, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.N.; Nunes, K.P.; Torres, F.S.; Cassoli, J.S.; Santos, D.M.; Almeida Fde, M.; Matavel, A.; Cruz, J.S.; Santos-Miranda, A.; Nunes, A.D.; et al. PnPP-19, a Synthetic and Nontoxic Peptide Designed from a Phoneutria nigriventer Toxin, Potentiates Erectile Function via NO/cGMP. J. Urol. 2015, 194, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Cordeiro, M.N.; Richardson, M.; Borges, M.N.; Diniz, S.O.; Cardoso, V.N.; Tostes, R.; De Lima, M.E.; Webb, R.C.; Leite, R. Nitric oxide-induced vasorelaxation in response to PnTx2-6 toxin from Phoneutria nigriventer spider in rat cavernosal tissue. J. Sex. Med. 2010, 7, 3879–3888. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Torres, F.S.; Borges, M.H.; Matavel, A.; Pimenta, A.M.; De Lima, M.E. New insights on arthropod toxins that potentiate erectile function. Toxicon 2013, 69, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Alewood, D.; Birinyi-Strachan, L.C.; Pallaghy, P.K.; Norton, R.S.; Nicholson, G.M.; Alewood, P.F. Synthesis and characterization of delta-atracotoxin-Ar1a, the lethal neurotoxin from venom of the Sydney funnel-web spider (Atrax robustus). Biochemistry 2003, 42, 12933–12940. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Mueller, A.; Israel, M.R.; Vetter, I. The pharmacology of voltage-gated sodium channel activators. Neuropharmacology 2017, 127, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingerd, J.S.; Mozar, C.A.; Ussing, C.A.; Murali, S.S.; Chin, Y.K.; Cristofori-Armstrong, B.; Durek, T.; Gilchrist, J.; Vaughan, C.W.; Bosmans, F.; et al. The tarantula toxin beta/delta-TRTX-Pre1a highlights the importance of the12 S–S voltage-sensor region for sodium channel subtype selectivity. Sci. Rep. 2017, 7, 974. [Google Scholar] [CrossRef] [PubMed]
- Leipold, E.; Hansel, A.; Borges, A.; Heinemann, S.H. Subtype specificity of scorpion beta-toxin Tz1 interaction with voltage-gated sodium channels is determined by the pore loop of domain 3. Mol. Pharmacol. 2006, 70, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.M.; Little, M.J.; Birinyi-Strachan, L.C. Structure and function of delta-atracotoxins: Lethal neurotoxins targeting the voltage-gated sodium channel. Toxicon 2004, 43, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Cologna, C.T.; Cremonez, C.M.; Mille, B.G.; Pucca, M.B.; Cuypers, E.; Arantes, E.C.; Tytgat, J. A gamut of undiscovered electrophysiological effects produced by Tityus serrulatus toxin 1 on NaV-type isoforms. Neuropharmacology 2015, 95, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Leipold, E.; Borges, A.; Heinemann, S.H. Scorpion beta-toxin interference with NaV channel voltage sensor gives rise to excitatory and depressant modes. J. Gen. Physiol. 2012, 139, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Rash, L.D.; Birinyi-Strachan, L.C.; Nicholson, G.M.; Hodgson, W.C. Neurotoxic activity of venom from the Australian eastern mouse spider (Missulena bradleyi) involves modulation of sodium channel gating. Br. J. Pharmacol. 2000, 130, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Gunning, S.J.; Chong, Y.; Khalife, A.A.; Hains, P.G.; Broady, K.W.; Nicholson, G.M. Isolation of delta-missulenatoxin-Mb1a, the major vertebrate-active spider delta-toxin from the venom of Missulena bradleyi (Actinopodidae). FEBS Lett. 2003, 554, 211–218. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Willow, M.; Howden, M.E.; Narahashi, T. Modification of sodium channel gating and kinetics by versutoxin from the Australian funnel-web spider Hadronyche versuta. Pflugers Arch. 1994, 428, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.M.; Little, M.J.; Tyler, M.; Narahashi, T. Selective alteration of sodium channel gating by Australian funnel-web spider toxins. Toxicon 1996, 34, 1443–1453. [Google Scholar] [CrossRef]
- Gilles, N.; Harrison, G.; Karbat, I.; Gurevitz, M.; Nicholson, G.M.; Gordon, D. Variations in receptor site-3 on rat brain and insect sodium channels highlighted by binding of a funnel-web spider delta-atracotoxin. Eur. J. Biochem. 2002, 269, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.L.; Matavel, A.; Peigneur, S.; Cordeiro, M.N.; Tytgat, J.; Diniz, M.R.; de Lima, M.E. Differential effects of the recombinant toxin PnTx4(5-5) from the spider Phoneutria nigriventer on mammalian and insect sodium channels. Biochimie 2016, 121, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Beress, L.; Tytgat, J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharmacol. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
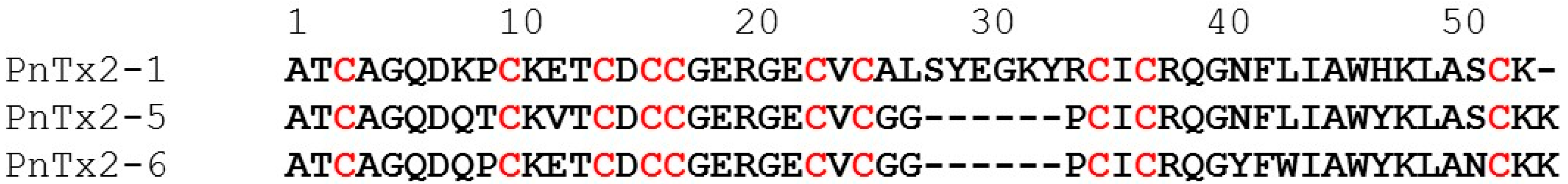

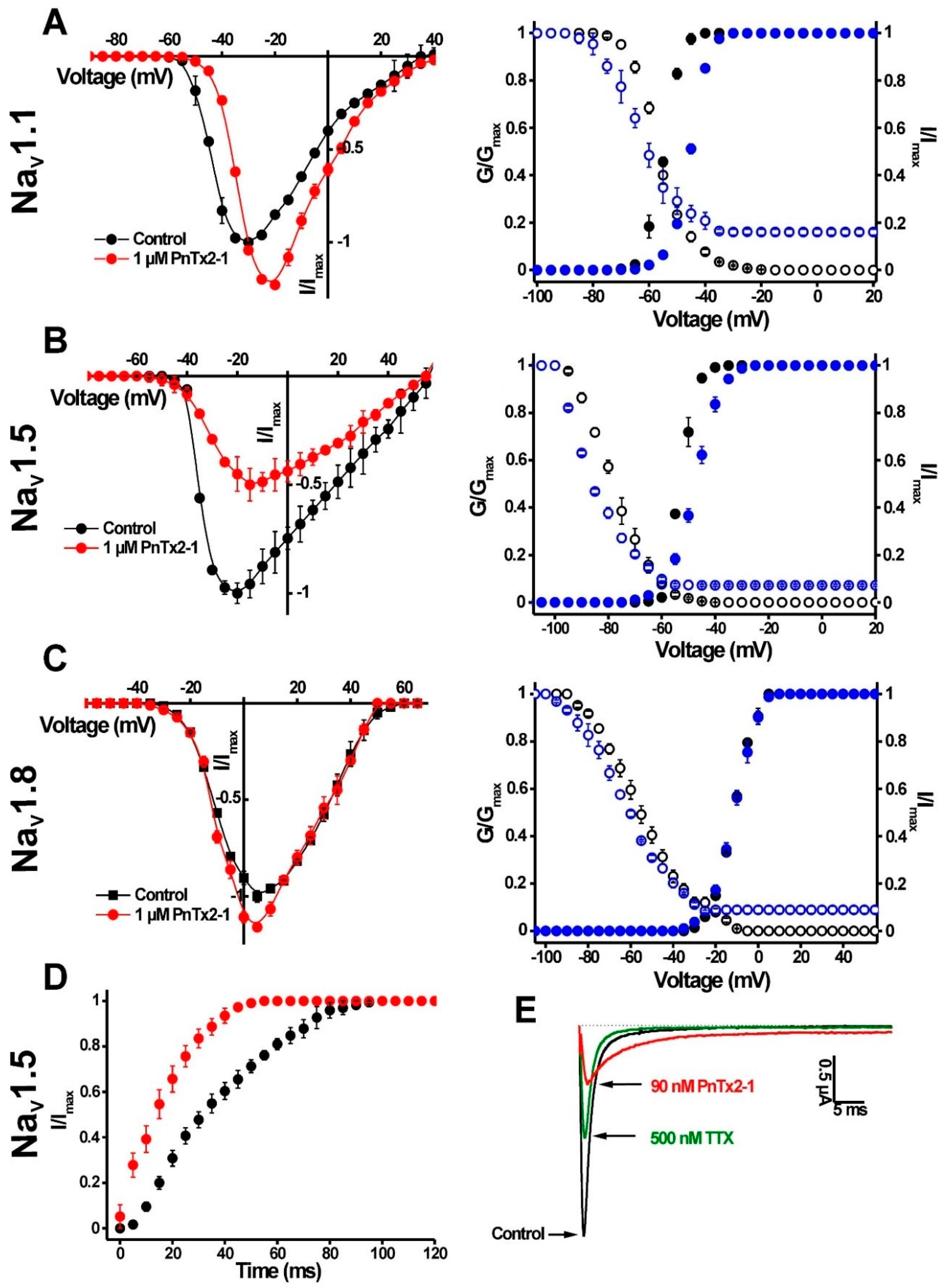
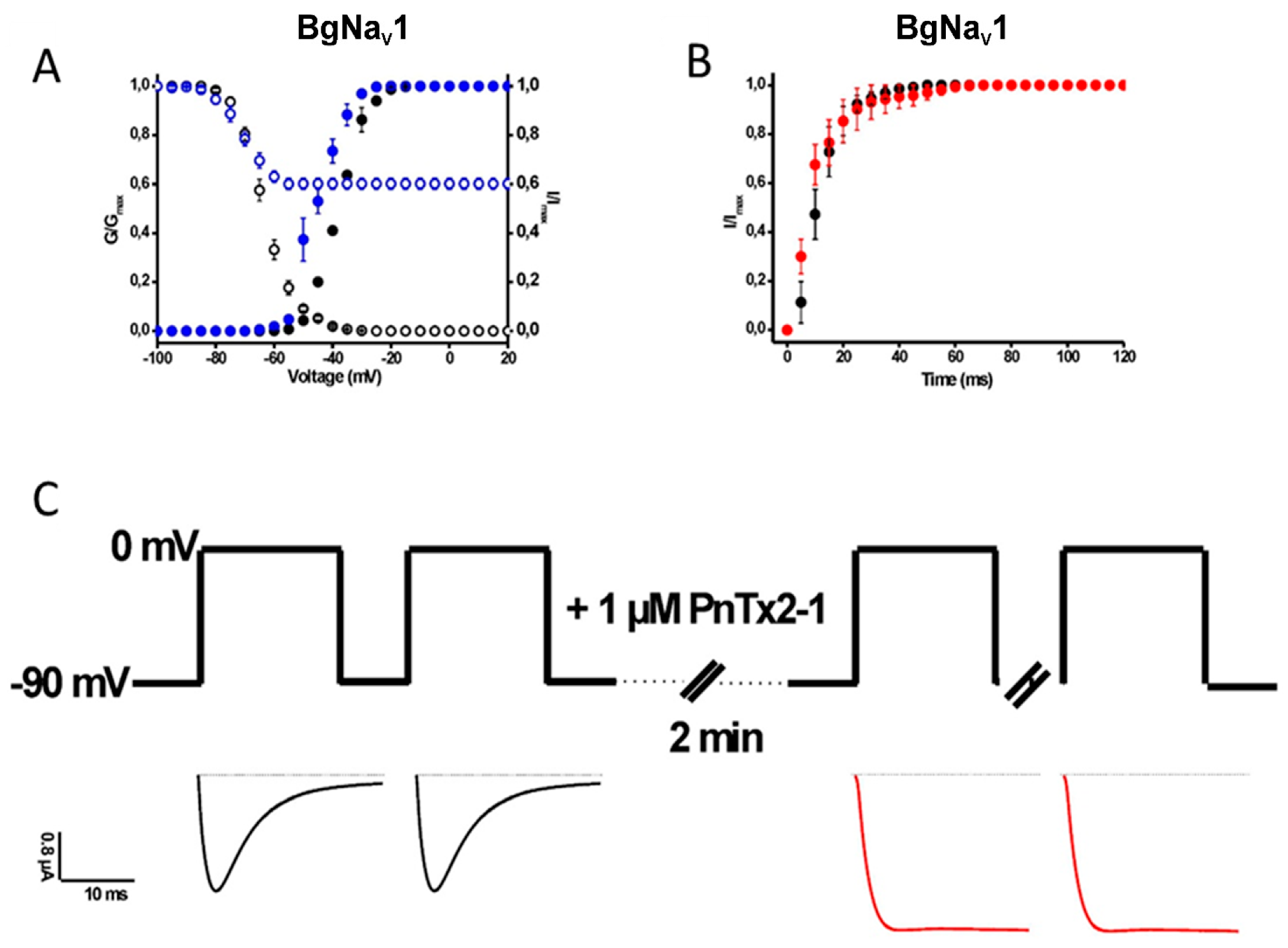

| Activation | Inactivation | |||
|---|---|---|---|---|
| V1/2 (mV) | Control | PnTx2-1 (1 µM) | Control | PnTx2-1 (1 µM) |
| NaV1.1 | −56.4 ± 0.2 | −63.4 ± 0.1 | −54.7 ± 0.1 | −45.3 ± 0.1 |
| NaV1.5 | −53.1 ± 0.1 | −45.3 ± 0.1 | −78.4 ± 0.2 | −87.5 ± 0.7 |
| NaV1.8 | −11.5 ± 0.1 | −11.4 ± 0.1 | −55.8 ± 0.4 | −63.9 ± 0.4 |
| BgNaV1 | −38.1 ± 0.1 | −45.8 ± 0.3 | −63.3 ± 0.3 | −70.7 ± 0.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peigneur, S.; Paiva, A.L.B.; Cordeiro, M.N.; Borges, M.H.; Diniz, M.R.V.; De Lima, M.E.; Tytgat, J. Phoneutria nigriventer Spider Toxin PnTx2-1 (δ-Ctenitoxin-Pn1a) Is a Modulator of Sodium Channel Gating. Toxins 2018, 10, 337. https://doi.org/10.3390/toxins10090337
Peigneur S, Paiva ALB, Cordeiro MN, Borges MH, Diniz MRV, De Lima ME, Tytgat J. Phoneutria nigriventer Spider Toxin PnTx2-1 (δ-Ctenitoxin-Pn1a) Is a Modulator of Sodium Channel Gating. Toxins. 2018; 10(9):337. https://doi.org/10.3390/toxins10090337
Chicago/Turabian StylePeigneur, Steve, Ana Luiza B. Paiva, Marta N. Cordeiro, Márcia H. Borges, Marcelo R. V. Diniz, Maria Elena De Lima, and Jan Tytgat. 2018. "Phoneutria nigriventer Spider Toxin PnTx2-1 (δ-Ctenitoxin-Pn1a) Is a Modulator of Sodium Channel Gating" Toxins 10, no. 9: 337. https://doi.org/10.3390/toxins10090337





