Current Advances in Immunological Studies on the Vespidae Venom Antigen 5: Therapeutic and Prophylaxis to Hypersensitivity Responses
Abstract
:1. Introduction
Venom
2. Antigen 5
2.1. Superfamily CAP
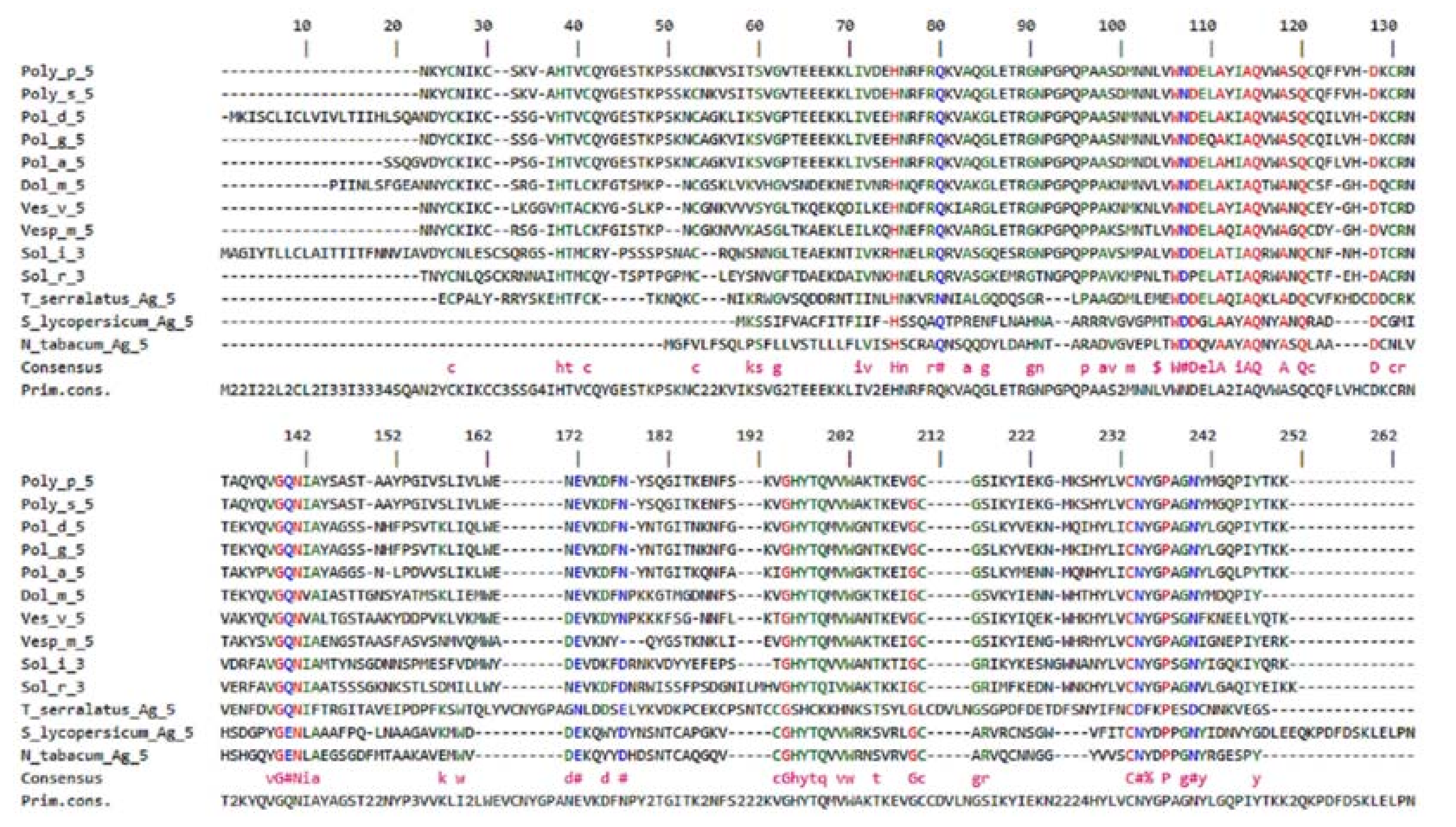
2.2. Isoforms of Ag 5
2.3. Immune Response to Allergens
2.4. Cross Reactivity
2.5. Potential of the Molecule Ag 5 in Immunotherapy
3. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeanne, R.L. Evolution of Social Behavior in the Vespidae. Annu. Rev. Entomol. 1980, 25, 371–396. [Google Scholar] [CrossRef]
- Pickett, K.; Wenzel, J. Phylogenetic analysis of the New World Polistes (Hymenoptera: Vespidae: Polistinae) using morphology and molecules. J. Kansas Entomol. Soc. 2004, 77, 742–760. [Google Scholar] [CrossRef]
- Johnson, B.R.; Borowiec, M.L.; Chiu, J.C.; Lee, E.K.; Atallah, J.; Ward, P.S. Phylogenomics resolves evolutionary relationships among ants, bees, and wasps. Curr. Biol. 2013, 23, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, W.; Beakes, D.E.; Huebner, S. Hymenoptera Venom Hypersensitivity Evaluation. J. Allergy Clin. Immunol. 2013, 131, AB26. [Google Scholar] [CrossRef]
- Casale, T.B.; Burks, A.W.; Solomon, C.G.; Casale, T.B.; Burks, A.W. Hymenoptera-Sting Hypersensitivity. N. Engl. J. Med. 2014, 370, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.G. Diagnostic methods for insect sting allergy. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Shafaghat, F.; Zwiener, R.D. Immunology of Bee Venom. Clin. Rev. Allergy Immunol. 2018, 54, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.B.K.K. New directions in diagnostic evaluation of insect allergy. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.C.F.F.; Wiezel, G.A.; Amorim, F.G.; Arantes, E.C. Arthropod venom Hyaluronidases: Biochemical properties and potential applications in medicine and biotechnology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Demain, J.G.; Minaei, A.A.; Tracy, J.M. Anaphylaxis and insect allergy. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Bilò, B.M.; Bonifazi, F. Epidemiology of insect-venom anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Moneret-Vautrin, A.; Scherer, K.; Lang, R.; Fernandez-Rivas, M.; Cardona, V.; Jutel, M.; Poziomkowska-Gesicka, I.; Papadopoulos, N.G.; Beyer, K.; et al. First European data from the network of severe allergic reactions (NORA). Allergy 2014, 69, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Srisong, H.; Daduang, S.; Lopata, A.L. Current advances in ant venom proteins causing hypersensitivity reactions in the Asia-Pacific region. Mol. Immunol. 2016, 69, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, A.; Justo-Jacomini, D.L.; de Lima Zollner, R.; Brochetto-Braga, M.R. Facing hymenoptera venom allergy: From natural to recombinant allergens. Toxins (Basel) 2015, 7, 2551–2570. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, C.; Miehe, U.; Treudler, R.; Kiess, W.; Prenzel, F. Long-term follow-up of children after venom immunotherapy: Low adherence to anaphylaxis guidelines. Int. Arch. Allergy Immunol. 2017, 172, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Schiener, M.; Graessel, A.; Ollert, M.; Schmidt-Weber, C.B.; Blank, S. Allergen-specific immunotherapy of Hymenoptera venom allergy—Also a matter of diagnosis. Hum. Vaccines Immunother. 2017, 13, 2467–2481. [Google Scholar] [CrossRef] [PubMed]
- Visitsunthorn, N.; Kijmassuwan, T.; Visitsunthorn, K.; Pacharn, P.; Jirapongsananuruk, O. Clinical characteristics of allergy to Hymenoptera stings. Pediatric Emergency Care 2017. [Google Scholar] [CrossRef] [PubMed]
- Van Vaerenbergh, M.; De Smet, L.; Rafei-Shamsabadi, D.; Blank, S.; Spillner, E.; Ebo, D.G.; Devreese, B.; Jakob, T.; de Graaf, D.C. IgE recognition of chimeric isoforms of the honeybee (Apis mellifera) venom allergen Api m 10 evaluated by protein array technology. Mol. Immunol. 2015, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ollert, M.; Blank, S. Anaphylaxis to insect venom allergens: Role of molecular diagnostics. Curr. Allergy Asthma Rep. 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Schiener, M.; Eberlein, B.; Moreno-Aguilar, C.; Pietsch, G.; Serrano, P.; McIntyre, M.; Schwarze, L.; Russkamp, D.; Biedermann, T.; Spillner, E.; et al. Application of recombinant antigen 5 allergens from seven allergy-relevant Hymenoptera species in diagnostics. Allergy 2017, 72, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W. Kreuzreaktionen zwischen den Giften von Hymenopteren unterschiedlicher Familien, Gattungen und Arten. Der Hautarzt 2014, 65, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Mauro, M.; Pravettoni, V.; Pucci, S. Hypersensitivity to Hymenoptera venom: Advances in diagnosis and implications for treatment. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jakob, T.; Rafei-Shamsabadi, D.; Spillner, E.; Müller, S. Diagnostics in Hymenoptera venom allergy: Current concepts and developments with special focus on molecular allergy diagnostics. Allergo J. Int. 2017, 26, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Jakob, T.; Müller, U.; Helbling, A.; Spillner, E. Component resolved diagnostics for hymenoptera venom allergy. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmer, W.; Focke, M.; Kolarich, D.; Dalik, I.; Götz, M.; Jarisch, R. Identification by immunoblot of venom glycoproteins displaying immunoglobulin E-binding N-glycans as cross-reactive allergens in honeybee and yellow jacket venom. Clin. Exp. Allergy 2004, 34, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Spillner, E.; Blank, S.; Jakob, T. Hymenoptera allergens: From venom to venome. Front. Immunol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Altmann, F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J. Int. 2016, 25, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habermann, E.; Neumann, W.P. Purification of phospholipase A of bee venom. Biochem. Z. 1957, 328, 465–473. [Google Scholar] [PubMed]
- Hoffman, D.R.; Sakell, R.H.; Schmidt, M. Sol i 1, the phospholipase allergen of imported fire ant venom. J. Allergy Clin. Immunol. 2005, 115, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.D.; Brown, S.G.; Chataway, T.K.; Davies, N.W.; Milne, R.W.; Aulfrey, S.J.; Heddle, R.J. Myrmecia pilosula (Jack Jumper) ant venom: Identification of allergens and revised nomenclature. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.F.M.; Palma, M.S.; Brochetto-Braga, M.R.; Malaspina, O.; Lazaretti, J.; Baldo, M.A. Biochemical properties and study of antigenic cross-reactivity between Africanized honey bee and wasp venom. J. Investig. Allergol. Clin. Immunol. 1994, 4, 37–41. [Google Scholar] [PubMed]
- De Oliveira, M.R.; Palma, M.S. Polybitoxins: A group of phospholipases A2 from the venom of the neotropical social wasp paulistinha (Polybia paulista). Toxicon 1998, 36, 189–199. [Google Scholar] [CrossRef]
- Costa, H.; Palma, M.S. Agelotoxin: A phospholipase A2 from the venom of the neotropical social wasp cassununga (Agelaia pallipes pallipes) (Hymenoptera-Vespidae). Toxicon 2000, 38, 1367–1379. [Google Scholar] [CrossRef]
- Santos, L.D.; Santos, K.S.; de Souza, B.M.; Arcuri, H.A.; Cunha-Neto, E.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Purification, sequencing and structural characterization of the phospholipase A1 from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae). Toxicon 2007, 50, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Kolarich, D.; Léonard, R.; Hemmer, W.; Altmann, F. The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. FEBS J. 2005, 272, 5182–5190. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.D.; Santos, K.S.; Pinto, J.R.A.; Dias, N.B.; Souza, B.M.; dos Santos, M.F.; Perales, J.; Domont, G.B.; Castro, F.M.; Kalil, J.E.; et al. Profiling the proteome of the venom from the social wasp Polybia paulista: A clue to understand the envenoming mechanism. J. Proteome Res. 2010, 9, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Riches, H.R.C. Hypersensitivity to bee venom. Bee World 1982, 63, 7–22. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Yamane, S.; Matsuura, M.; Starr, C.K. Hornet venoms: Lethalities and lethal capacities. Toxicon 1986, 24, 950–954. [Google Scholar] [CrossRef]
- Schmidt, J.H. Biochemistry of insect venoms. Annu. Rev. Entomol. 1982, 27, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Dotimas, E.M.; Hamid, K.R.; Hider, R.C.; Ragnarsson, U. Isolation and structure analysis of bee venom mast cell degranulating peptide. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1987, 3, 285–293. [Google Scholar] [CrossRef]
- Banks, B.E.C.; Shipolini, R.A. Chemistry and Pharmacology of Honey-Bee Venom. In Venoms of the Hymenoptera: Biochemical, Pharmacological and Behaviour Aspects; Piek, T., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1986; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=xBQlBQAAQBAJ&oi=fnd&pg=PA329&dq=Chemistry+and+pharmacology+of+honey-bee+venom.+In:+Venoms+of+the+Hymenoptera&ots=CC3St9vfZ0&sig=2E2gP77xzN0Cfwy4XhwVCksfiig (accessed on 16 May 2018).
- Hoffman, D.R.; Jacobson, R.S. Allergens in hymenoptera venom XXVII: Bumblebee venom allergy and allergens. J. Allergy Clin. Immunol. 1996, 97, 812–821. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Brochetto-Braga, M.R.; Ruberti, M.; Ternero, M.L.L.; Gobbi, N.; Silva, G.P. A comparative study of protein and enzymatic activity in venoms of some common wasps (Hymenoptera: Vespidae) from São Paulo State. Sociobiology 2004, 44, 271–282. [Google Scholar]
- Rawlings, N.D. Protease families, evolution and mechanism of action. In Proteases Structure and Function; Springer: Vienna, Austria, 2013; pp. 1–36. [Google Scholar]
- King, T.P.; Sobotka, A.K.; Alagon, A.; Kochoumian, L.; Lichtenstein, L.M. Protein allergens of white-faced hornet, yellow hornet, and yellow jacket venoms. Biochemistry 1978, 17, 5165–5174. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R. Allergens in hymenoptera venom XV: The immunologic basis of vespid venom cross-reactivity. J. Allergy Clin. Immunol. 1985, 75, 611–613. [Google Scholar] [CrossRef]
- Aparecido Dos Santos-Pinto, J.R.; Delazari Dos Santos, L.; Arcuri, H.A.; Ribeiro Da Silva Menegasso, A.; Pêgo, P.N.; Santos, K.S.; Castro, F.M.; Kalil, J.E.; De-Simone, S.G.; Palma, M.S. B-cell linear epitopes mapping of antigen-5 allergen from Polybia paulista wasp venom. J. Allergy Clin. Immunol. 2015, 135, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yasuhara, T.; Uzu, S.; Wakamatsu, K.; Miyazawa, T.; Fukuda, K.; Tsukamoto, Y. Wasp venom peptides; wasp kinins, new cytotrophic peptide families and their physico-chemical properties. Peptides 1985, 6, 425–430. [Google Scholar] [CrossRef]
- Pirpignani, M.L.; Rivera, E.; Hellman, U.; Biscoglio de Jiménez Bonino, M. Structural and immunological aspects of Polybia scutellaris Antigen 5. Arch. Biochem. Biophys. 2002, 407, 224–230. [Google Scholar] [CrossRef]
- Pantera, B.; Hoffman, D.R.; Carresi, L.; Cappugi, G.; Turillazzi, S.; Manao, G.; Severino, M.; Spadolini, I.; Orsomando, G.; Moneti, G.; et al. Characterization of the major allergens purified from the venom of the paper wasp Polistes gallicus. Biochim. Biophys. Acta Gen. Subj. 2003, 1623, 72–81. [Google Scholar] [CrossRef]
- An, S.; Chen, L.; Wei, J.-F.; Yang, X.; Ma, D.; Xu, X.; Xu, X.; He, S.; Lu, J.; Lai, R. Purification and characterization of two new allergens from the venom of Vespa magnifica. PLoS ONE 2012, 7, e31920. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.M.; Roelants, K.; O’Bryan, M.K. The CAP superfamily: Cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—Roles in reproduction, cancer, and immune defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R. Hymenoptera venom allergens. Clin. Rev. Allergy Immunol. 2006, 30, 109–128. [Google Scholar] [CrossRef]
- Milne, T.J.; Abbenante, G.; Tyndall, J.D.A.; Halliday, J.; Lewis, R.J. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J. Biol. Chem. 2003, 278, 31105–31110. [Google Scholar] [CrossRef] [PubMed]
- Padavattan, S.; Schmidt, M.; Hoffman, D.R.; Marković-Housley, Z. Crystal Structure of the Major Allergen from Fire Ant Venom, Sol i 3. J. Mol. Biol. 2008, 383, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Asojo, O.A.; Goud, G.; Dhar, K.; Loukas, A.; Zhan, B.; Deumic, V.; Liu, S.; Borgstahl, G.E.O.; Hotez, P.J. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J. Mol. Biol. 2005, 346, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Cascone, O.; Amaral, V.; Ferrara, P.; Vita, N.; Guillemot, J.C.; Díaz, L.E. Purification and characterization of two forms of antigen 5 from Polybia scutellaris venom. Toxicon 1995, 33, 659–665. [Google Scholar] [CrossRef]
- Vinzón, S.E.; Marino-Buslje, C.; Rivera, E.; Biscoglio de Jiménez Bonino, M. A naturally occurring hypoallergenic variant of vespid antigen 5 from Polybia scutellaris venom as a candidate for allergen-specific immunotherapy. PLoS ONE 2012, 7, e41351. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.; King, T.P.; Mirza, O.; Monsalve, R.I.; Meno, K.; Ipsen, H.; Larsen, J.N.; Gajhede, M.; Spangfort, M.D. Major venom allergen of yellow jackets, ves v 5: Structural characterization of a pathogenesis-related protein superfamily. Proteins Struct. Funct. Genet. 2001, 45, 438–448. [Google Scholar] [CrossRef] [PubMed]
- dos Santos-Pinto, J.R.A.; dos Santos, L.D.; Andrade Arcuri, H.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Using Proteomic Strategies for Sequencing and Post-Translational Modifications Assignment of Antigen-5, a Major Allergen from the Venom of the Social Wasp Polybia paulista. J. Proteome Res. 2014, 13, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Shefler, I.; Panasoff, J.; Paitan, Y.; Confino-Cohen, R. Immunotherapy with commercial venoms is efficacious for anaphylactic reactions to vespa orientalis stings. Int. Arch. Allergy Immunol. 2013, 161, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tanno, L.K.; Calderon, M.; Papadopoulos, N.G.; Demoly, P. Mapping hypersensitivity/allergic diseases in the International Classification of Diseases (ICD)-11: Cross-linking terms and unmet needs. Clin. Transl. Allergy 2015, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Justo Jacomini, D.L.; Gomes Moreira, S.M.; Campos Pereira, F.D.; Zollner, R.D.L.; Braga, M.R.B. Reactivity of IgE to the allergen hyaluronidase from Polybia paulista (Hymenoptera, Vespidae) venom. Toxicon 2014, 82, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R. Broad perspectives of allergen specific immunotherapy. Hum. Vaccines Immunother. 2017, 13, 2385–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Li, Y.; Yu, J.; Feng, L.; Hou, S.; Liu, Y. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS ONE 2013, 8, e54841. [Google Scholar] [CrossRef] [PubMed]
- Robbe, P.; Draijer, C.; Borg, T.R.; Luinge, M.; Timens, W.; Wouters, I.M.; Melgert, B.N.; Hylkema, M.N. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L358–L367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.J.; Tsang, T.M.; Qiu, Y.; Dayrit, J.K.; Freij, J.B.; Huffnagle, G.B.; Olszewski, M.A. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio 2013, 4, e00264–13. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.; Martins, C.; Borrego, L.M. Regulatory B cells and allergy: Uncovering the link. J. Investig. Allergol. Clin. Immunol. 2017, 27, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Zingarelli, B.; Hake, P.W.; O’Connor, M.; Denenberg, A.; Kong, S.; Aronow, B.J. Absence of poly(ADP-ribose)polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol. Med. 2003, 9, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Martignago, I.; Incorvaia, C.; Ridolo, E. Preventive actions of allergen immunotherapy: The facts and the effects in search of evidence. Clin. Mol. Allergy 2017, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Seismann, H.; Blank, S.; Braren, I.; Greunke, K.; Cifuentes, L.; Grunwald, T.; Bredehorst, R.; Ollert, M.; Spillner, E. Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of α-1,3-core fucosylation. Mol. Immunol. 2010, 47, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Brehler, R.; Grundmann, S.; Stöcker, B. Cross-reacting carbohydrate determinants and hymenoptera venom allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, S.; Omenaas, E.; Oryszczyn, M.P. Allergy markers in respiratory epidemiology. Eur. Respir. J. 2001, 17, 773–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, A.; Miehe, M.; Jabs, F.; Seismman, H.; Romani Fernandes, L.G.; de Lima Zollner, R.; Jakob, T.; Brochetto-Braga, M.R.; Spillner, E. Venoms of Neotropical wasps lack cross-reactive carbohydrate determinants enabling reliable protein-based specific IgE determination. J. Allergy Clin. Immunol. 2018, 141, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Neu, C.; Hasche, D.; Bantleon, F.I.; Jakob, T.; Spillner, E. Polistes species venom is devoid of carbohydrate-based cross-reactivity and allows interference-free diagnostics. J. Allergy Clin. Immunol. 2013, 131, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.R. Recombinant hymenoptera venom allergens. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 570–576. [Google Scholar] [CrossRef]
- Müller, U.R. Insect Venoms. In Anaphylaxis; Karger: Basel, Switzerland, 2010; pp. 141–156. [Google Scholar]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential properties of venom peptides and proteins in solitary vs. social hunting wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Caruso, B.; Bonadonna, P.; Bovo, C.; Melloni, N.; Lombardo, C.; Senna, G.; Lippi, G. Wasp venom allergy screening with recombinant allergen testing. Diagnostic performance of rPol d 5 and rVes v 5 for differentiating sensitization to Vespula and Polistes subspecies. Clin. Chim. Acta 2016, 453, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Van Vaerenbergh, M.; Cardoen, D.; Formesyn, E.M.; Brunain, M.; Van Driessche, G.; Blank, S.; Spillner, E.; Verleyen, P.; Wenseleers, T.; Schoofs, L.; et al. Extending the honey bee venome with the antimicrobial peptide apidaecin and a protein resembling wasp antigen 5. Insect Mol. Biol. 2013, 22, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, L.; Vogel, M.; Stadler, M.; Truffer, R.; Rohner, E.; Stadler, B.M. Sensitization to wasp venom does not induce autoantibodies leading to infertility. Mol. Immunol. 2008, 45, 3775–3785. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Villalba, M.; Coscia, M.R.; Hoffman, D.R.; King, T.P. Sequence analysis and antigenic cross-reactivity of a venom allergen, antigen 5, from hornets, wasps, and yellow jackets. J. Immunol. 1993, 150, 2823–2830. [Google Scholar] [PubMed]
- Korošec, P.; Valenta, R.; Mittermann, I.; Čelesnik, N.; Šilar, M.; Zidarn, M.; Košnik, M. High sensitivity of CAP-FEIA rVes v 5 and rVes v 1 for diagnosis of Vespula venom allergy. J. Allergy Clin. Immunol. 2012, 129, 1406–1408. [Google Scholar] [CrossRef] [PubMed]
- Vinzón, S.E.; Pirpignani, M.L.; Nowicki, C.; De Jimènez Bonino, M.B. Molecular cloning and expression in Pichia pastoris of a hypoallergenic antigen 5. Protein Expr. Purif. 2010, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, B.; Krischan, L.; Darsow, U.; Ollert, M.; Ring, J. Double positivity to bee and wasp venom: Improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J. Allergy Clin. Immunol. 2012, 130, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Francischetti, I.M.B. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.G.; Pham, V.M.; Garfield, M.K.; Francischetti, I.M.B.; Ribeiro, J.M.C. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2002, 32, 1101–1122. [Google Scholar] [CrossRef]
- Mans, B.J.; Andersen, J.F.; Francischetti, I.M.B.; Valenzuela, J.G.; Schwan, T.G.; Pham, V.M.; Garfield, M.K.; Hammer, C.H.; Ribeiro, J.M.C. Comparative sialomics between hard and soft ticks: Implications for the evolution of blood-feeding behavior. Insect Biochem. Mol. Biol. 2008, 38, 42–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, X.; Eichwald, V.; Daeffler, L.; Landry, Y. Activation of subunits of Gi2 and Gi3 proteins by basic secretagogues induces exocytosis through Phospholipase C and arachidonate release through Phospholipase C in Mast Cells. J. Immunol. 2001, 167, 4805–4813. [Google Scholar] [CrossRef] [PubMed]
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Matthews, S.A.; Turner, H.; Kinet, J.P. Signal transduction by the high-affinity immunoglobulin E receptor Fc epsilon RI: Coupling form to function. Adv. Immunol. 2000, 76, 325–355. [Google Scholar] [PubMed]
- Koshikawa, N.; Nakamura, T.; Tsuchiya, N.; Isaji, M.; Yasumitsu, H.; Umeda, M.; Miyazaki, K. Purification and identification of a novel and four known serine proteinase inhibitors secreted by human glioblastoma cells. J. Biochem. 1996, 119, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kischnick, S.; Weber, B.; Verdino, P.; Keller, W.; Sanders, E.A.; Anspach, F.B.; Fiebig, H.; Cromwell, O.; Suck, R. Bacterial fermentation of recombinant major wasp allergen Antigen 5 using oxygen limiting growth conditions improves yield and quality of inclusion bodies. Protein Expr. Purif. 2006, 47, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bohle, B.; Zwolfer, B.; Fischer, G.F.; Seppala, U.; Kinaciyan, T.; Bolwig, C.; Spangfort, M.D.; Ebner, C. Characterization of the human T cell response to antigen 5 from Vespula vulgaris (Ves v 5). Clin. Exp. Allergy 2005, 35, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Macchia, D.; Cortellini, G.; Mauro, M. Vespa crabro immunotherapy versus Vespula-venom immunotherapy in Vespa crabro allergy: A comparison study in field re-stings. World Allergy Organ. J. 2018, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Vovolis, V.; Mikos, N.; Koutsostathis, N. Successful venom immunotherapy to paper wasp, in IgE-venom negative patient. Allergol. Immunopathol. 2011, 39, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Przybilla, B.; Ruëff, F. Hymenoptera venom allergy. J. Dtsch. Dermatol. Ges. 2010, 8, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Biló, B.M.; Rueff, F.; Mosbech, H.; Bonifazi, F.; Oude-Elberink, J.N.G. Diagnosis of Hymenoptera venom allergy. Allergy 2005, 60, 1339–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutel, M.; Akdis, M.; Blaser, K.; Akdis, C.A. Mechanisms of allergen specific immunotherapy—T-cell tolerance and more. Allergy 2006, 61, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Linhart, B.; Valenta, R. Molecular design of allergy vaccines. Curr. Opin. Immunol. 2005, 17, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Bazon, M.L.; Perez-Riverol, A.; Dos Santos-Pinto, J.R.A.; Fernandes, L.G.R.; Lasa, A.M.; Justo-Jacomini, D.L.; Palma, M.S.; de Lima Zollner, R.; Brochetto-Braga, M.R. Heterologous expression, purification and immunoreactivity of the antigen 5 from Polybia paulista wasp venom. Toxins (Basel) 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazon, M.L.; Silveira, L.H.; Simioni, P.U.; Brochetto-Braga, M.R. Current Advances in Immunological Studies on the Vespidae Venom Antigen 5: Therapeutic and Prophylaxis to Hypersensitivity Responses. Toxins 2018, 10, 305. https://doi.org/10.3390/toxins10080305
Bazon ML, Silveira LH, Simioni PU, Brochetto-Braga MR. Current Advances in Immunological Studies on the Vespidae Venom Antigen 5: Therapeutic and Prophylaxis to Hypersensitivity Responses. Toxins. 2018; 10(8):305. https://doi.org/10.3390/toxins10080305
Chicago/Turabian StyleBazon, Murilo Luiz, Lais Helena Silveira, Patricia Ucelli Simioni, and Márcia Regina Brochetto-Braga. 2018. "Current Advances in Immunological Studies on the Vespidae Venom Antigen 5: Therapeutic and Prophylaxis to Hypersensitivity Responses" Toxins 10, no. 8: 305. https://doi.org/10.3390/toxins10080305




