1. Introduction
At present, the detection of toxins is one of the main tasks for environmental science, security, agriculture, the food industry, and medicine. There is particular interest in detection of mycotoxins, products of the metabolism of numerous fungi species, which appear to have toxic, carcinogenic, and hormone-disruptive effects in humans [
1]. Worldwide legislation sets quite strict limits on mycotoxin content in food and feed, typically at the ppb (part-per-billion) concentration level [
2], which makes the detection of small mycotoxin molecules (with typical molecular weight in hundreds of daltons) a difficult task. Existing high-tech detection methods such as HPLC and mass spectroscopy can provide the required sensitivity, but such methods are expensive and time-consuming. Therefore, there is a great demand for development of biosensors for toxin detection. Highly sensitive optical immunosensors are leading in this development [
3].
Our previous research exploiting the method of total internal reflection ellipsometry (TIRE) combined with direct immunoassay showed high sensitivity (in the sub-ppb range) for detection of different mycotoxins [
4,
5,
6]. The use of a planar waveguide (PW) operating as a polarization interferometer (PI) [
7] is a logical continuation of this work toward the development of portable sensor devices. The advantages of PW PI devices, due to their thousands of reflections of light, were demonstrated in [
7,
8], which may lead to the development of highly sensitive optical biosensors capable of label-free detection of toxins [
3,
6]. Several successful biosensors based on planar waveguides have been demonstrated recently. An inhibition sensor array based on PW optrodes using enzymes as bioreceptors was capable of detecting traces (in the sub-ppb concentration range) of heavy metals and pesticides in water [
9]. The mainstream development of optical PW-based sensor devices lies in the use of Mach–Zahender (MZ) interferometers [
10,
11,
12,
13,
14] and ring resonators [
15,
16], both approaches having demonstrating remarkable refractive index sensitivity around 7000 to 8000 rad/refractive index unit (RIU) [
3] and versatility in their application. The development of a fully integrated all-silicon MZ biosensor [
17,
18,
19,
20] is particularly attractive and may lead to fabrication of portable, highly sensitive optical biosensors suitable for in-field or point-of-care detection of analytes of interest.
The main purpose of this work was to further explore the use of a much simpler PW sensor design (as compared to MZ-based devices) operating as a polarization interferometer with a view toward developing portable and highly sensitive devices capable of detecting low-molecular-weight molecules such as mycotoxins, particularly ochratoxin A, in very low concentrations in the ppt range.
2. Interferometric Sensor System
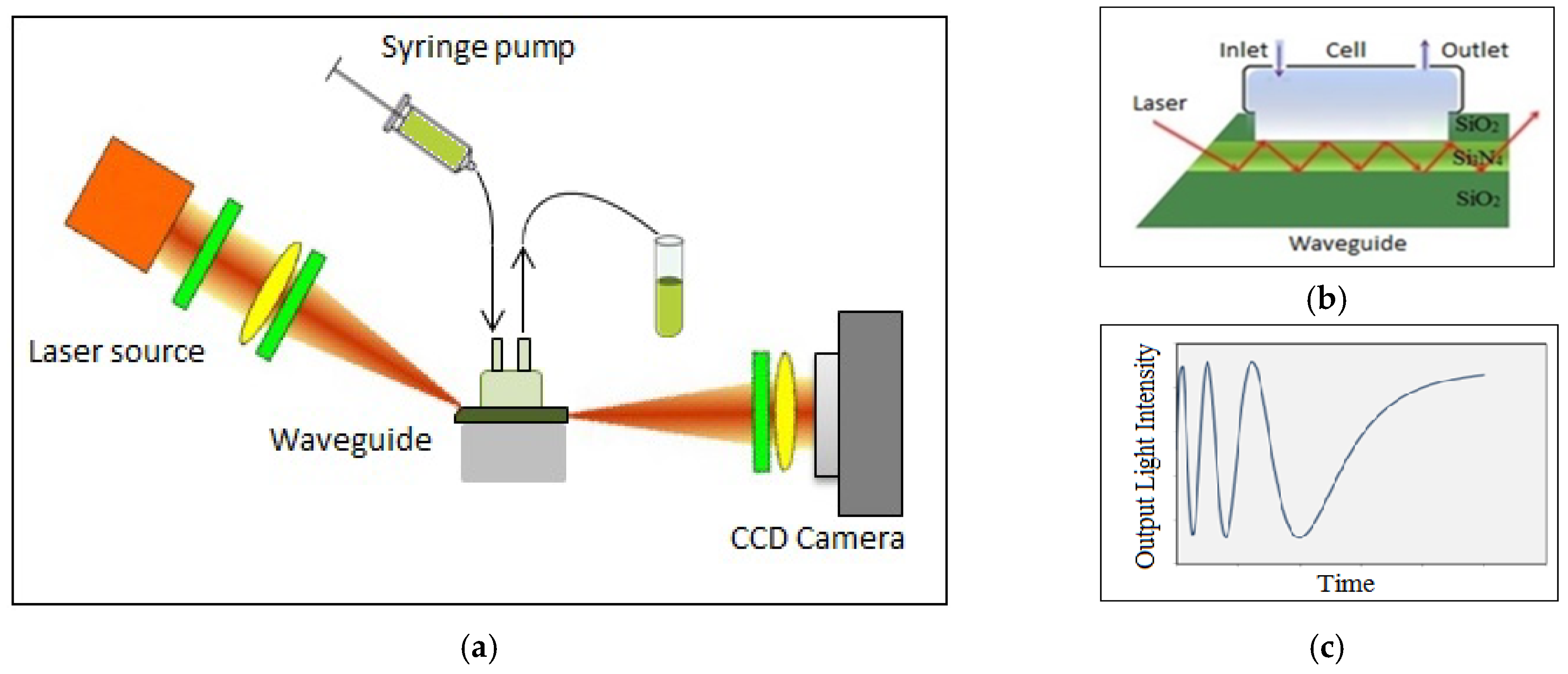
The planar waveguide structures (shown in
Figure 1a) were produced on silicon wafers using standard microelectronic processes and consisted of a thin (200 nm) layer of
sandwiched between much thicker (3 μm) layers of
. Due to the large difference in the refractive indices of
core (n = 2) and
cladding (n = 1.46), the light propagates at an angle of 47° and thus experiences about 800 reflections per mm according to calculations based on the Goos–Hänchen effect [
21].
In the experimental polarization interferometer (PI) setup in
Figure 1b, a 635 nm light from a fan-beam laser diode was first made circularly polarized by λ/4 plate, then focused to a narrow strip using a semicylindrical lens and coupled to the waveguide through the slanted edge. At the other side of the waveguide, the light was collected with a charge-coupled device (CCD) array. A polarizer placed in front of the CCD camera allows the conversion of a phase shift between p- and s-components of polarized light into variations of light intensity (
Figure 1c).
To monitor biochemical reactions, a window was etched in the top
layer, which brings the liquid sample in contact with the waveguide core. The reaction cell is sealed against the window and has inlet and outlet tubes to allow the injection of required liquids into the cell, and thus the adsorption of biomolecules on the surface of
. Any changes in either the refractive index or the thickness of the adsorbed molecular layer affects mostly the p-component of polarized light, while the s-component acts as a reference, resulting in a multiperiodic output signal (
Figure 1c):
where
is the phase shift between the p- and s-components of polarized light.
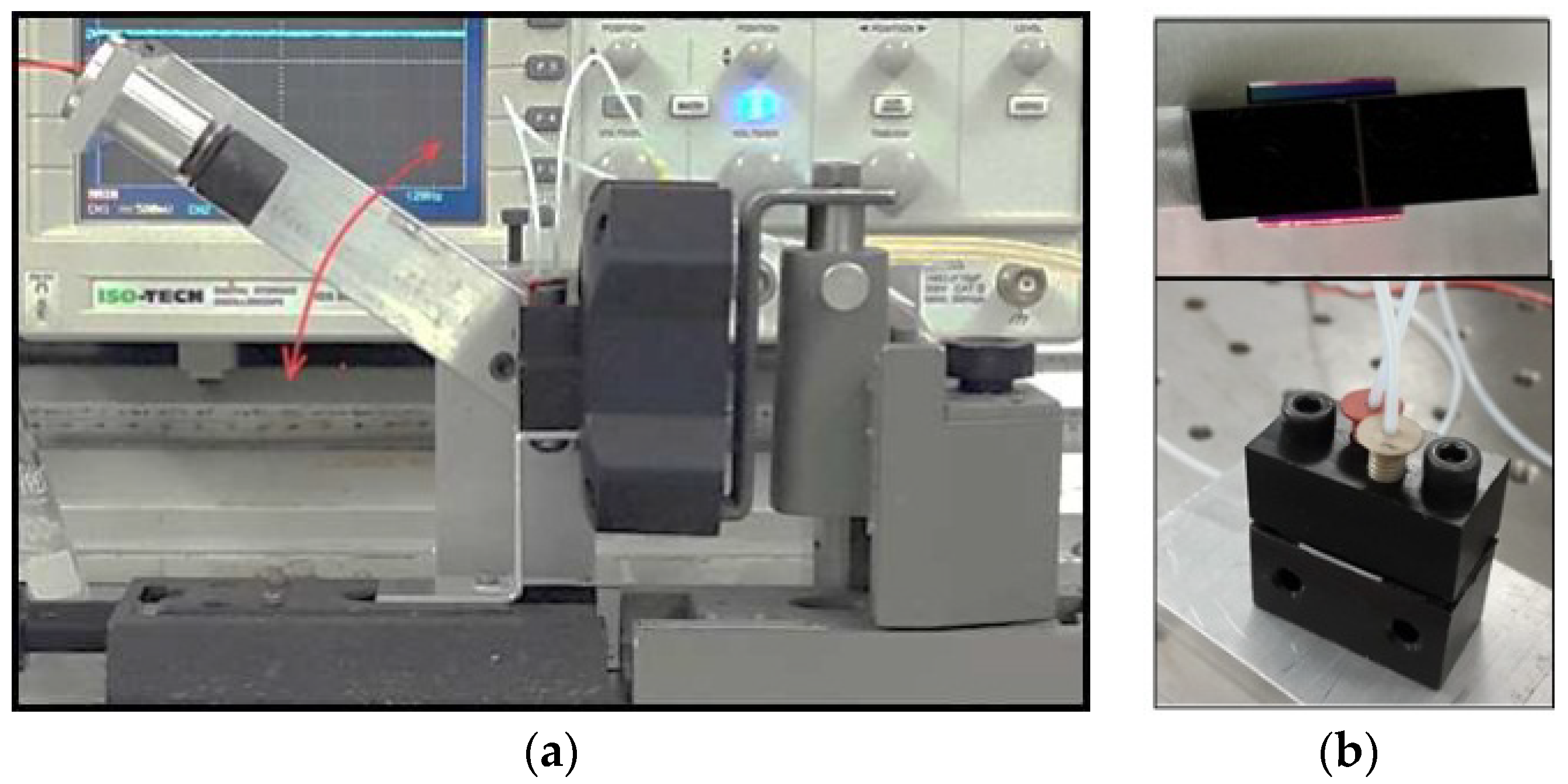
The photographs in
Figure 2 show a general view of the PI biosensor setup (2a) and the cell with the inserted waveguide (2b) and the light coupling through the waveguide slanted edge. A Thorlabs (UK) LC100–Smart Line Camera was connected to a PC; SPLICCO dedicated software was used to record the output signals.
3. Testing the Polarization Interferometer
The sensitivity of the waveguide was initially tested by injecting NaCl aqueous solution of different concentrations into the cell. Multiperiodic output signals were recorded, and the number of periods of signal oscillations was roughly estimated from these waveforms. The results of these tests are presented in
Figure 3.
The refractive index sensitivity (RIS) of PW sensors can be estimated as a gradient of the above linear dependence:
where
N is the number of periods of oscillations and
is the change in the refractive index of liquid medium. The obtained average refractive index sensitivity was around 5200 radians per refractive index unit (RIU), which was more than double compared to the earlier version of the PI experimental setup (Nabok, 2017) and close to the values reported for MZ PW sensors (Nabok, 2016). The achieved sensitivity is much higher than that in other traditional optical methods such as TIRE (total internal reflection ellipsometry) or SPR (surface plasmon resonance).
4. Immunosensing Tests on Detection of Ochratoxin A
To prepare the system for detection of mycotoxin molecules, we used electrostatic immobilization of proteins. First, a positively charged layer of poly-allylamine hydrochloride (PAH) was deposited, followed by adsorption of protein A molecules, which are negatively charged, in Tris-HCl buffer, pH 7. Finally, monoclonal antibodies to ochratoxin A (in Tris-HCl buffer) were bound to protein A, and the sensor was ready for detection of ochratoxin A (OTA). All the chemicals used were purchased from Sigma-Aldrich, Dorset, UK.
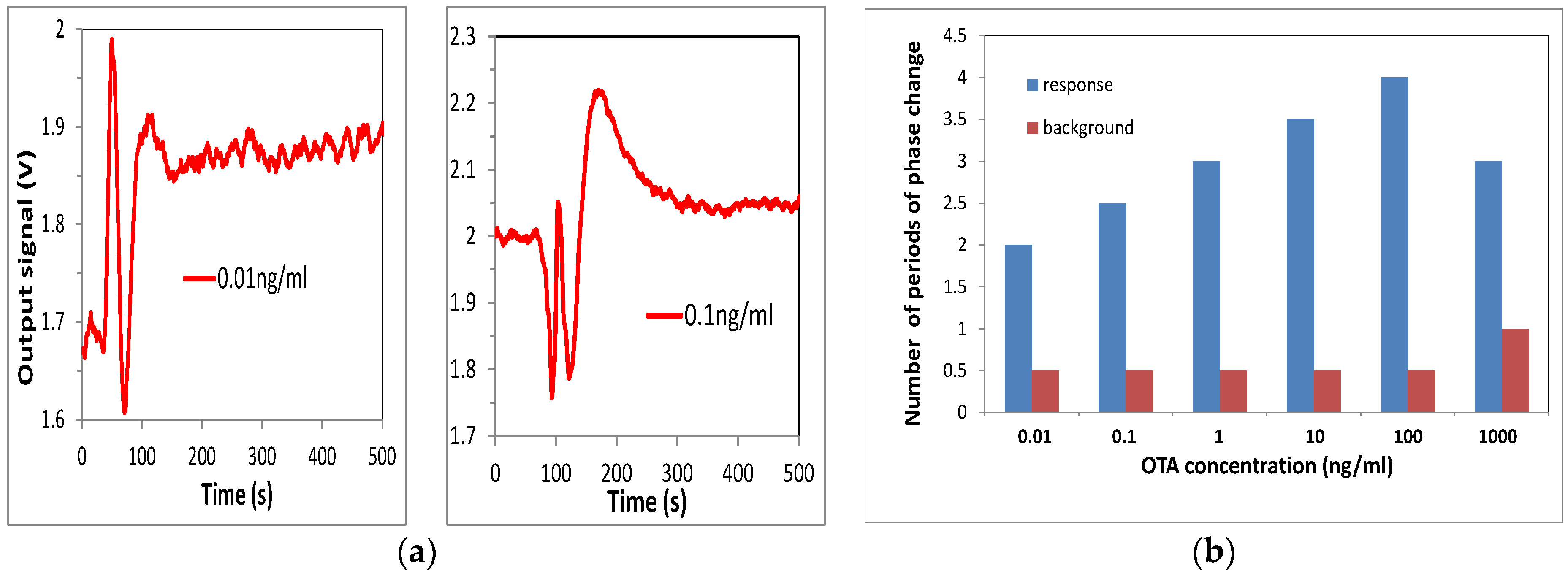
Biosensing tests were performed by injection of OTA solution in water of different concentrations starting from the lowest: 0.01 ng/mL, 0.1 ng/mL, 1 ng/mL, 10 ng/mL, 100 ng/mL, and 1000 ng/mL. The sensor responses were recorded, and the typical responses to 0.01 ng/mL and 0.1 ng/mL of OTA are shown in
Figure 4a.
The results of these tests are summarized in
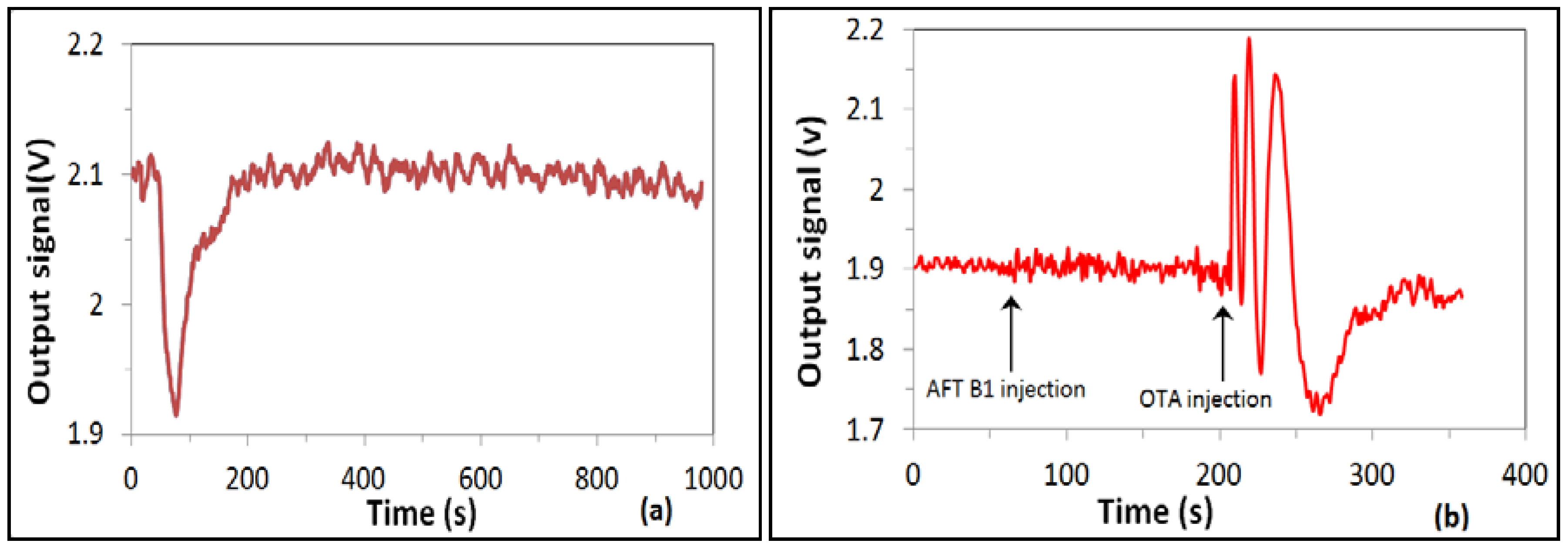
Figure 4b as the dependence of the phase shift against the concentration of OTA. The sensor response increased in a wide range of concentrations from 0.01 to 100 ng/mL, then decreased at a high concentration of 1 g/mL due to the saturation of bioreceptors. The results obtained are similar to those reported earlier for detection of aflatoxin B1 (Nabok, 2017), though the RIS value and thus the signal clarity were much better. Such biosensing tests were repeated several times; while the waveforms looked slightly different each time because of different initial phase conditions, the total values of a phase shift looked similar, with an accuracy of about 10%. Control test measurements were carried out after each step of OTA binding by purging of about 1 mL of pure Tris-HCl buffer in order to wash out nonspecifically bound OTA molecules. Such tests typically result in a half-period of phase change (see
Figure 5a), which corresponds well to observations in MZ-based biosensors [
14]. Corresponding background phase changes are also given in
Figure 4b. After subtracting the π radians background level, a phase shift corresponding to binding of 0.01 ng/mL of OTA is about one period (or 2π radians). This means that the detection limit could be at least one order of magnitude smaller, thus reaching ppt level or even below, an absolutely remarkable outcome, especially considering the direct immunoassay format used.
5. Conclusions and Future Work
The experimental setup of a planar polarization interferometer was developed and tested. After several stages of development, a refractive index sensitivity of 5200 rad/RIU was achieved. A series of biosensing experiments for detecting ochratoxin A in direct immunoassay with specific antibodies were successful; the biosensor was capable of detecting 0.01 ng/mL of ochratoxin A. The work is currently under way. Further development will focus on (i) improving the planar waveguide sensor design using photolithography to make several narrow waveguide channels for simultaneous detection of several mycotoxins, and (ii) developing the data acquisition system using NI card and LabView software. Significant improvements in sensor performance and sensitivity are expected in the near future.
Author Contributions
A.A.-J. (experimental work, data analysis), A.N. (supervision, data analysis, writing), R.J. (funding), A.H. (supervision, data acquisition), A.T. (supervision, data analysis), E.T. (experimental work, immunoassay), A.S. (funding, immunoassay, discussion of results).
Funding
This work was funded by NATO Science for Peace and Security Program through the project NUKR.SFPP 984637. PhD scholarship was provided by the University of Babylon College of Sciences.
Acknowledgments
The authors acknowledge support from the NATO Science for Peace and Security Program through the project NUKR.SFPP 984637. A.M. Al-Jawdah thanks the University of Babylon College of Sciences for PhD scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peraica, M.; Radic, B.; Lucic, A.; Pavlovic, M. Toxic Effects of Mycotoxins in Humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Worldwide Mycotoxin Regulations-Romer Labs. 2016. Available online: https://www.romerlabs.com/en/knowledge-center/knowledge-library/articles/news/worldwide-mycotoxin-regulations/ (accessed on 2 July 2018).
- Nabok, A. Comparative Studies on Optical Biosensors for Detection of Bio-Toxins. In Advanced Sciences and Technologies in Security Applications, Biosensors for Security and Bioterrorism Applications; Nikolelis, D.P., Nikoleli, G.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 491–508. [Google Scholar]
- Nabok, A.; Tsargorodskaya, A.; Hassan, A.K.; Starodub, N.F. Total internal reflection ellipsometry and SPR detection of low molecular weight environmental toxins. Appl. Surf. Sci. 2005, 246, 381–386. [Google Scholar] [CrossRef]
- Nabok, A.; Tsargorodskaya, A. The Method of Total Internal Reflection Ellipsometry for Thin Film Characterisation and Sensing. Thin Solid Films 2008, 516, 8993–9001. [Google Scholar] [CrossRef]
- Nabok, A.; Tsargorodskaya, A.; Mustafa, M.K.; Szekacs, I.; Starodub, N.F.; Szekacs, A. Detection of low molecular weight toxins using optical phase detection techniques. Sens. Actuators B Chem. 2011, 154, 232–237. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Jawdah, A.M.; Tsargorodska, A. Development of planar waveguide-based immunosensor for detection of low molecular weight molecules such as mycotoxins. Sens. Actuators B Chem. 2017, 247, 975–980. [Google Scholar] [CrossRef]
- Shirshov, Y.M.; Snopok, B.A.; Samoylov, A.V.; Kiyanovskij, A.P.; Venger, E.F.; Nabok, A.V.; Ray, A.K. Analysis of the response of planar interferometer to molecular layer formation: Fibrinogen adsorption on silicon nitride surface. Biosens. Bioelectron. 2001, 16, 381–390. [Google Scholar] [CrossRef]
- Nabok, A.; Haron, S.; Ray, A.K. Optical enzyme sensors based upon silicon planar waveguide coated with composite polyelectrolyte film. Appl. Surf. Sci. 2004, 238, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Lechuga, L.M. Optical biosensors. Compr. Anal. Chem. 2005, 44, 209–250. [Google Scholar]
- Zinoviev, K.E.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Integrated bimodal waveguide interferometric biosensor for label-free analysis. J. Lightw. Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef]
- Dante, S.; Duval, D.; Sepulveda, B.; Gonzales-Guerrero, A.B.; Sendra, J.R.; Lechuga, L.M. All-optical phase modulation for integrated interferometric biosensor. Opt. Exp. 2012, 20, 7195–7205. [Google Scholar] [CrossRef] [PubMed]
- Gavela, A.F.; García, D.G.; Ramirez, J.C.; Lechuga, L.M. Last advances in silicon-based optical biosensors. Sensors 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, L.G.; Huertas, C.S.; Lechuga, L.M. Prospects of optical biosensors for emerging label-free RNA analysis. Trends Anal. Chem. 2016, 80, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Fan, X. Optical ring resonators for biochemical and chemical sensing. Anal. Bioanal. Chem. 2011, 399, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, K.; Kakabakos, S.E.; Petrou, P.S.; Ruf, H.H. A monolithic silicon optoelectronic transducer as a real-time affinity biosensor. Anal. Chem. 2004, 76, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, K.; Petrou, P.S.; Kakabakos, S.E.; Yannoukakos, D.; Contopanagos, H.; Knoll, T.; Velten, T.; DeFazio, M.; Schiavoe, L.; Passamano, M.; et al. Fully integrated monolithic optoelectronic transducer for real-time protein and DNA detection The NEMOSLAB approach. Biosens. Bioelectron. 2010, 26, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Kitsara, M.; Misiakos, K.; Raptis, I.; Makarona, E. Integrated optical frequency-resolved Mach-Zehnder interferometers for label-free affinity sensing. Opt. Exp. 2010, 18, 8193–8206. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, K.; Raptis, I.; Makarona, E.; Botsialas, A.; Salapatas, A.; Oikonomou, P. All-silicon monolithic Mach-Zehnder interferometer as a refractive index and bio-chemical sensor. Opt. Exp. 2014, 22, 26803–26813. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chan, C.T.; Chen, H. Goos-Hanchen effect in epsilon-near-zero metamaterials. Sci. Rep. 2015, 5, 8681. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).