Bee Venom Phospholipase A2 Alleviate House Dust Mite-Induced Atopic Dermatitis-Like Skin Lesions by the CD206 Mannose Receptor
Abstract
:1. Introduction
2. Results
2.1. bvPLA2 Treatment Alleviated DFE/DNCB-Induced Ear Thickness and AD-Like Symptoms
2.2. bvPLA2 Reduced Serum IgE in DFE/DNCB-Treated Mice
2.3. bvPLA2 Abrogated AD-Related Th1 and Th2 Cytokine Production via CD206
2.4. bvPLA2 Is Associated with Treg Induction through the CD206 Receptor
2.5. bvPLA2 Decreased DFE/DNCB-Induced Epidermal and Dermal Thickness and Infiltration of Inflammatory Cells Depending on CD206
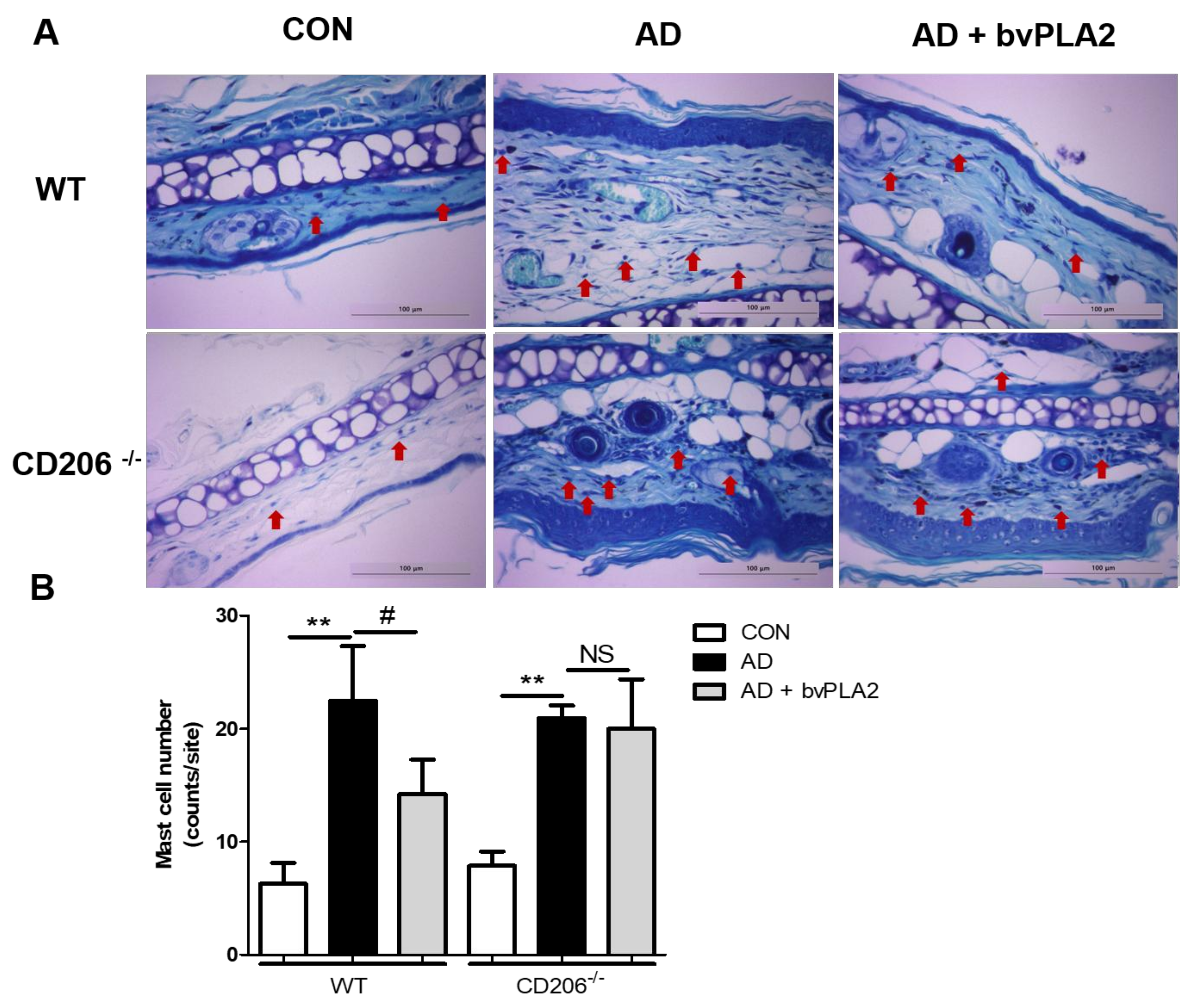
2.6. bvPLA2 Blocked Mast Cell Infiltration in DFE/DNCB-Induced AD via CD206
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Reagents
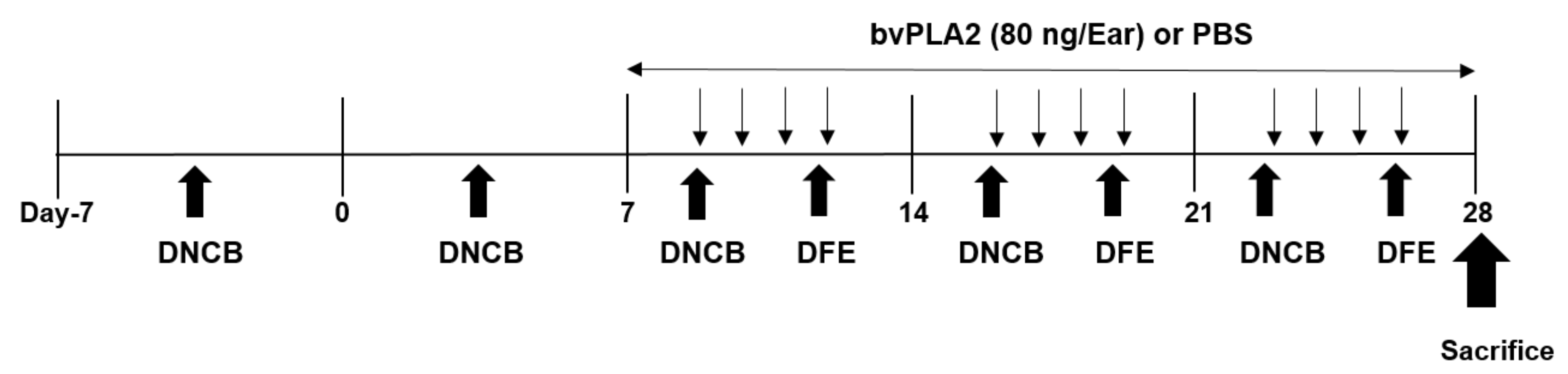
4.3. Experimental Protocol
4.4. Measurement of Serum Immunoglobulin E (IgE)
4.5. Assessment of Th1 and Th2 Cytokine Levels in Mouse Ear
4.6. Western Blot Assay
4.7. Histological Analysis
4.8. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, S.; Reynolds, N.J. Atopic and non-atopic eczema. BMJ 2006, 332, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Berke, R.; Singh, A.; Guralnick, M. Atopic dermatitis: An overview. Am. Fam. Phys. 2012, 86, 35–42. [Google Scholar]
- Silverberg, J.I.; Kleiman, E.; Lev-Tov, H.; Silverberg, N.B.; Durkin, H.G.; Joks, R.; Smith-Norowitz, T.A. Association between obesity and atopic dermatitis in childhood: A case-control study. J. Allergy Clin. Immunol. 2011, 127, 1180–1186, e1181. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Chung, B.Y.; Kim, H.O.; Park, C.W. Clinical Features of Atopic Dermatitis in Adults Are Different according to Onset. J. Korean Med. Sci. 2017, 32, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Boguniewicz, M.; Leung, D.Y.M.; Novak, N.; Simons, F.E.R.; Weidinger, S.; Akdis, M.; Eigenman, P.; Lipozencic, J.K.; Platts-Mills, T.A.E.; et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology American Academy of Allergy, Asthma and Immunology PRACTALL Consensus Report. J. Allergy Clin. Immunol. 2006, 118, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Novak, N. New insights into the mechanism and management of allergic diseases: Atopic dermatitis. Allergy 2009, 64, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Walling, H.W.; Swick, B.L. Update on the management of chronic eczema: New approaches and emerging treatment options. Clin. Cosmet. Investig. Dermatol. 2010, 3, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Chamlin, S.L.; Kao, J.; Frieden, I.J.; Sheu, M.Y.; Fowler, A.J.; Fluhr, J.W.; Williams, M.L.; Elias, P.M. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: Changes in barrier function provide a sensitive indicator of disease activity. J. Am. Acad. Dermatol. 2002, 47, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Baek, H.; Kang, M.; Kim, N.; Lee, S.Y.; Bae, H. Bee Venom Phospholipase A2 Ameliorates House Dust Mite Extract Induced Atopic Dermatitis Like Skin Lesions in Mice. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.J.; Mendez-Lnocencio, J.I.; Omidvar, B.; Omidvar, J.; Santilli, J.; Nielsen, H.S., Jr.; Pavot, A.P.; Richert, J.R.; Bellanti, J.A. A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy Asthma Proc. 2005, 26, 470–476. [Google Scholar] [PubMed]
- Chen, J.; Lariviere, W.R. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog. Neurobiol. 2010, 92, 151–183. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A. Venom therapy in multiple sclerosis. Neuropharmacology 2007, 53, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.W.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Bee and wasp venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, A.K.; Franklin, R.M.; Adkinson, N.F., Jr.; Valentine, M.; Baer, H.; Lichtenstein, L.M. Allergy to insect stings. II. Phospholipase A: The major allergen in honeybee venom. J. Allergy Clin. Immunol. 1976, 57, 29–40. [Google Scholar] [CrossRef]
- Dennis, E.A.; Rhee, S.G.; Billah, M.M.; Hannun, Y.A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991, 5, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Frattini, A.; Loffredo, S.; Del Prete, A.; Sozzani, S.; Marone, G.; Triggiani, M. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur. J. Immunol. 2006, 36, 1938–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.B.; Miele, L.; Pattabiraman, N. Phospholipase A2 enzymes: Regulation and physiological role. Biochem. Pharmacol. 1994, 48, 1–10. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.S.; Yoon, H.; Kang, G.H.; Hwang, D.S.; Kim, S.K.; Chung, H.S.; et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baek, H.; Jung, K.H.; Lee, G.; Lee, H.; Kang, G.H.; Lee, G.; Bae, H. Bee venom phospholipase A2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory T cells. Immun. Inflamm. Dis. 2015, 3, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell. Immunol. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y. Infection in atopic dermatitis. Curr. Opin. Pediatr. 2003, 15, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.D. Atopic diseases of childhood. Curr. Opin. Pediatr. 2002, 14, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Mizoguchi, E.; Oettgen, H.; Bhan, A.K.; Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 1999, 103, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q.; Naseer, T.; Minshall, E.M.; Song, Y.L.; Boguniewicz, M.; Leung, D.Y. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J. Allergy Clin. Immunol. 1996, 98, 225–231. [Google Scholar] [CrossRef]
- Choi, J.K.; Kim, S.H. Rutin suppresses atopic dermatitis and allergic contact dermatitis. Exp. Biol. Med. 2013, 238, 410–417. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.; Choi, W.; Bae, H. Bee Venom Phospholipase A2 Alleviate House Dust Mite-Induced Atopic Dermatitis-Like Skin Lesions by the CD206 Mannose Receptor. Toxins 2018, 10, 146. https://doi.org/10.3390/toxins10040146
Shin D, Choi W, Bae H. Bee Venom Phospholipase A2 Alleviate House Dust Mite-Induced Atopic Dermatitis-Like Skin Lesions by the CD206 Mannose Receptor. Toxins. 2018; 10(4):146. https://doi.org/10.3390/toxins10040146
Chicago/Turabian StyleShin, Dasom, Won Choi, and Hyunsu Bae. 2018. "Bee Venom Phospholipase A2 Alleviate House Dust Mite-Induced Atopic Dermatitis-Like Skin Lesions by the CD206 Mannose Receptor" Toxins 10, no. 4: 146. https://doi.org/10.3390/toxins10040146




