Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1
Abstract
:1. Introduction
2. Results
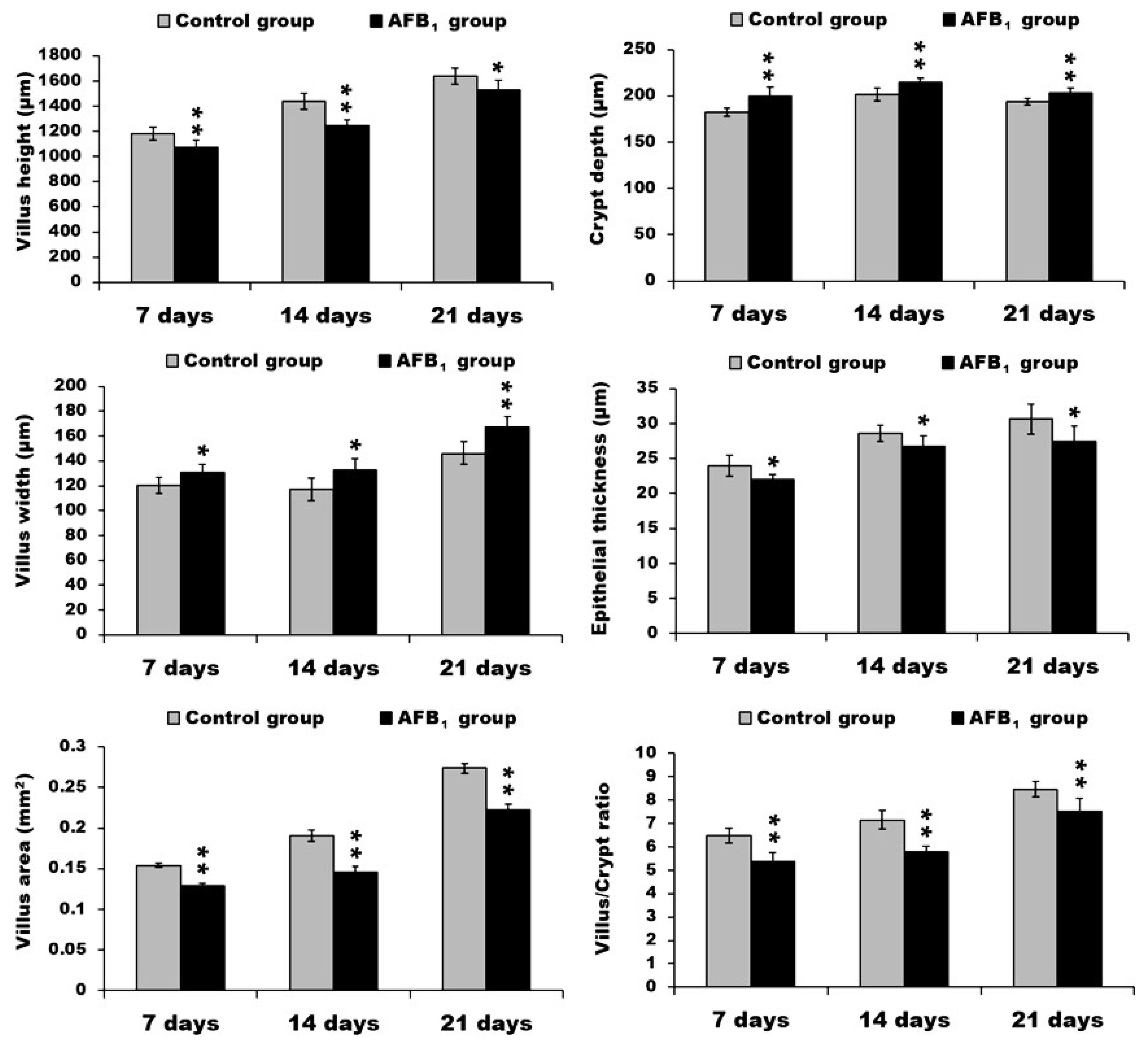
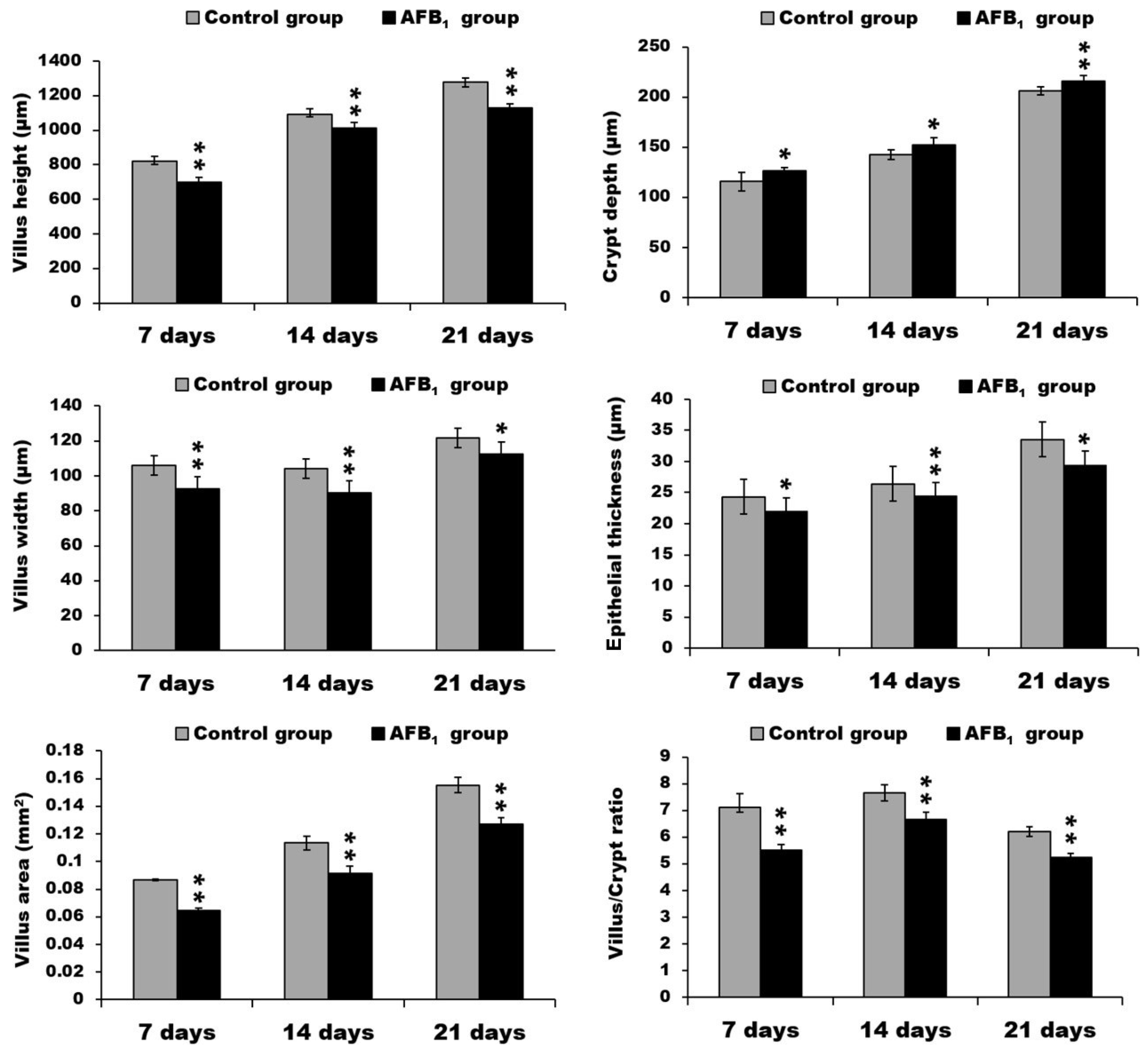
2.1. Morphological Measurements in the Small Intestine
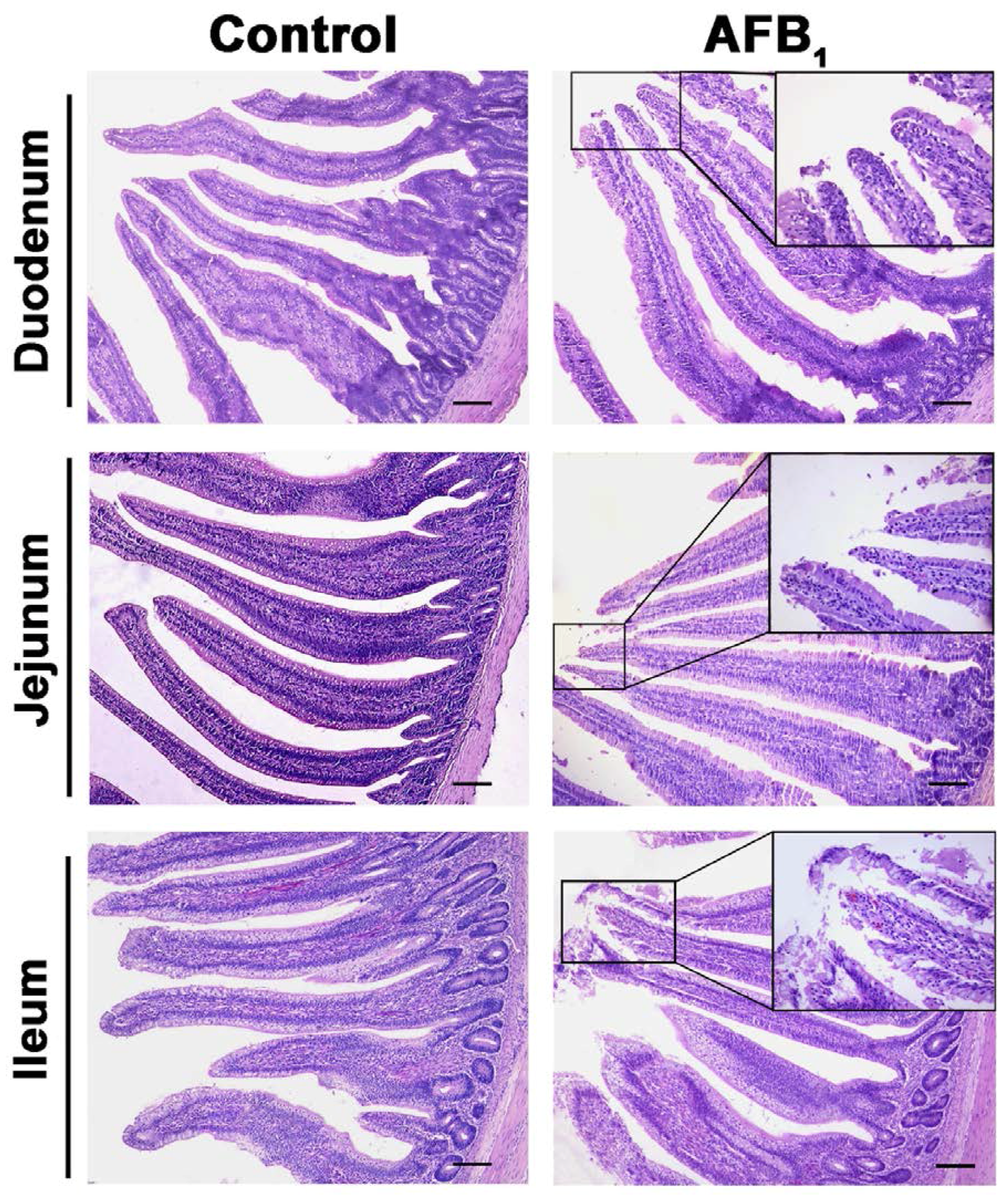
2.2. Histopathological Analysis
2.3. Ultrastructure Changes
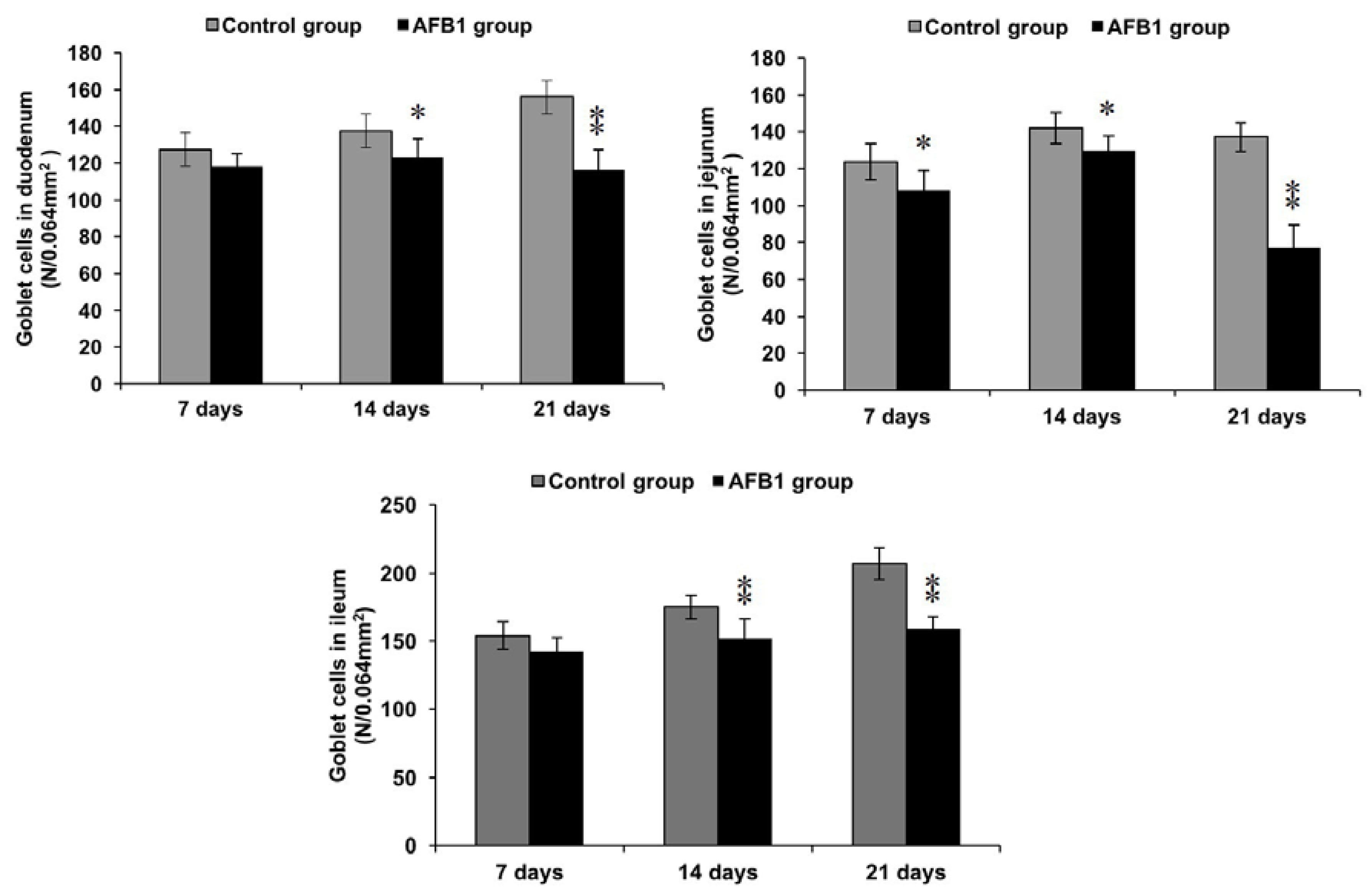
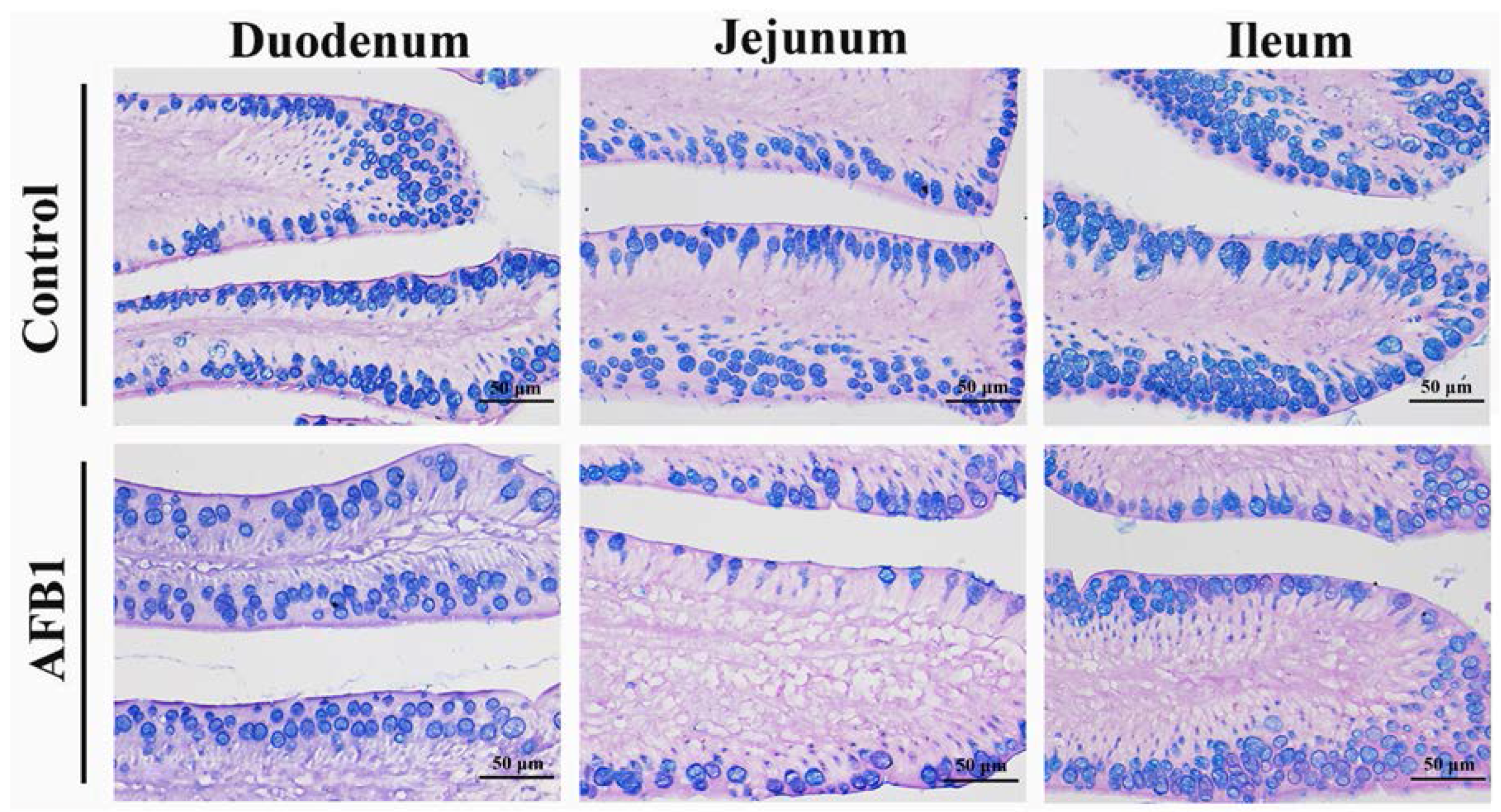
2.4. Number of Goblet Cells Shown by Alcian Blue/PAS
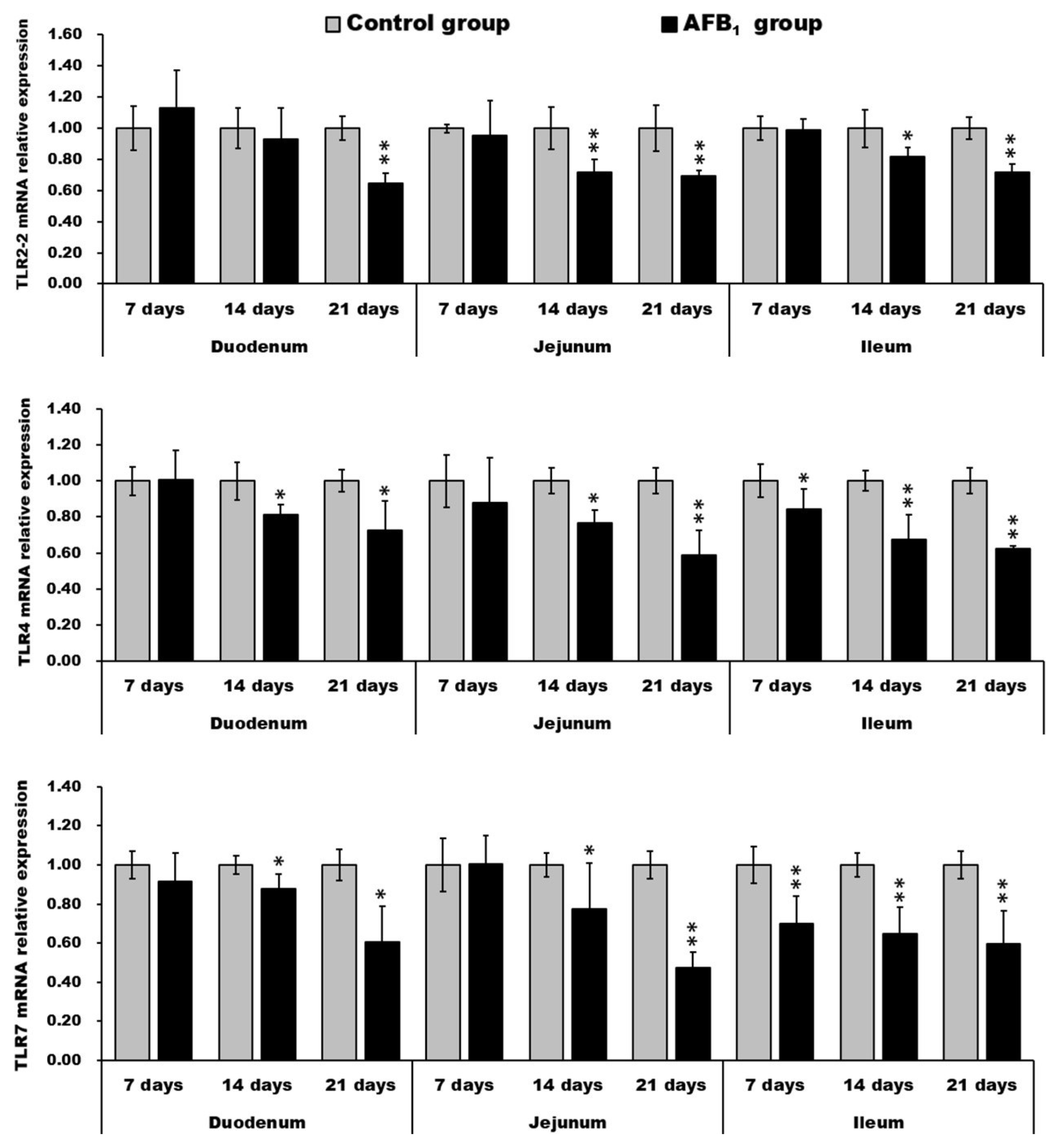
2.5. mRNA Expression of TLR2-2, TLR-4 and TLR-7
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Groups
5.2. Diets
5.3. Histopathological Observation and Microscopic Analyses
5.4. Transmission Electron Microscope Observation
5.5. Alcian Blue/Periodic Acid-Schiff (PAS) Stain
5.6. qRT-PCR
5.7. Statistical Analysis
Reference
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murphy, P.A.; Hendrich, S.; Landgren, C.; Bryant, C.M. Food mycotoxins: An update. J. Food Sci. 2006, 71, 51–65. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Garcia, H.S. Effect of oral supplementation of Lactobacillus reuteri in reduction of intestinal absorption of aflatoxin B1 in rats. J. Basic Microbiol. 2011, 51, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Xun, W. The emerging epidemic of environmental cancers in developing countries. Ann. Oncol. 2009, 20, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Teniola, O.D.; Addo, P.A.; Brost, I.M.; Färber, P.; Jany, K.D.; Alberts, J.F.; van Zyl, W.H.; Steyn, P.S.; Holzapfel, W.H. Degradation of aflatoxin B1 by cell-free extracts of Rhodococcus erythropolis and Mycobacterium fluoranthenivorans sp. nov. DSM44556(T). Int. J. Food Microbiol. 2005, 105, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.V.; Vasanthi, S.; Rao, B.S.; Rao, R.N.; Rao, V.S.; Nagaraja, K.V.; Bai, R.G.; Prasad, C.A.K.; Vanchinathan, S.; Roy, R.; et al. Aflatoxin B1 contamination in maize samples collected from different geographical regions of India—A multicentre study. Food Addit. Contam. 1997, 14, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Bababunmi, E.A.; Uwaifo, A.O.; Bassir, O. Hepatocarcinogens in Nigerian foodstuffs. World Rev. Nutr. Diet. 1978, 28, 188–209. [Google Scholar] [PubMed]
- Shivachandra, S.B.; Sah, R.L.; Singh, S.D.; Kataria, J.M.; Manimaran, K. Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Vet. Res. Commun. 2003, 27, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, K.; Chen, J.; Fang, J.; Cui, H.; Zuo, Z.; Deng, J.; Chen, Z.; Geng, Y.; Lai, W. Aflatoxin B1 affects apoptosis and expression of Bax, Bcl-2, and Caspase-3 in thymus and bursa of fabricius in broiler chickens. Environ. Toxicol. 2015, 30, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [PubMed]
- De Kivit, S.; Tobin, M.C.; Forsyth, C.B.; Keshavarzian, A.; Landay, A.L. Regulation of intestinal immune responses through TLR activation: Implications for pro- and prebiotics. Front. Immunol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjöberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Peng, X.; Fang, J.; Cui, H.; Yu, Z.; Chen, Z. Effects of aflatoxin B1 on T-cell subsets and mRNA expression of cytokines in the intestine of broilers. Int. J. Mol. Sci. 2015, 16, 6945–6959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fang, J.; Peng, X.; Cui, H.; Yu, Z. Effect of aflatoxin B1 on IgA+ cell number and immunoglobulin mRNA expression in the intestine of broilers. Immunopharmacol. Immunotoxicol. 2015, 37, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kana, J.R.; Teguia, A.; Choumboue, J.T. The evaluation of activated dietary charcoal from Canarium schweinfurthii Engl. seed and maize cob as toxin binder in broiler chickens. Adv. Anim. Biosci. 2010, 1, 467–468. [Google Scholar] [CrossRef]
- Yunus, A.W.; Ghareeb, K.; Abd-El-Fattah, A.A.; Twaruzek, M.; Böhm, J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult. Sci. 2011, 90, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Grozeva, N.; Valchev, I.; Hristov, T.; Lazarov, L.; Nikolov, Y. Histopathological changes in small intestines of broiler chickens with experimental aflatoxicosis. Agric. Sci. Technol. 2015, 7, 319–323. [Google Scholar]
- Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Alonso-Debolt, M. Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 1999, 78, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Malvandi, A.M.; Mehrzad, J.; Saleh-Moghaddam, M. Biologically relevant doses of mixed aflatoxins B and G up-regulate MyD88, TLR2, TLR4 and CD14 transcripts in human PBMCs. Immunopharmacol. Immunotoxicol. 2013, 35, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K. NF-κB activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, J.; Peng, X.; Fang, J.; Chen, K.; Yang, H. Effect of aflatoxin B1 pathological changes of immune organs in broilers. Acta Vet. Zootech. Sin. 2015, 46, 1447–1454. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Yuan, S.; Peng, X.; Fang, J.; Wang, F.; Cui, H.; Chen, Z.; Yuan, J.; Geng, Y. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicol. Ind. Health 2016, 32, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Peng, X.; Fang, J.; Cui, H.; Zuo, Z.; Chen, Z. Effects of aflatoxin B1 exposure and sodium selenite supplementation on the histology, cell proliferation, and cell cycle of jejunum in broilers. Biol. Trace Elem. Res. 2014, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Pistol, G.C.; Motiu, M.; Pelinescu, D. Induction of pro-inflammatory gene expression by Escherichia coli and mycotoxin zearalenone contamination and protection by a lactobacillus mixture in porcine IPEC-1 cells. Toxicon 2015, 97, 53. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [PubMed]
- Seeboth, J.; Solinhac, R.; Oswald, I.P.; Guzylack-Piriou, L. The fungal T-2 toxin alters the activation of primary macrophages induced by TLR-agonists resulting in a decrease of the inflammatory response in the pig. Vet. Res. 2012, 43, 35. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.E.; Brian, L.F. Dellmann’s Textbook of Veterinary Histology; Blackwell Publishing Professional: Ames, IA, USA, 2006; ISBN 0781741483. [Google Scholar]
- Hernández, F.; García, V.; Madrid, J.; Orengo, J.; Catalá, P.; Megías, M.D. Effect of formic acid on performance, digestibility, intestinal histomorphology and plasma metabolite levels of broiler chickens. Br. Poult. Sci. 2006, 47, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Geyra, A.; Uni, Z.; Sklan, D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci. 2001, 80, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Aboutalebi, N. Toxic effects of aflatoxin B1 on duodenum tissue. J. Am. Sci. 2013, 9, 115–117. [Google Scholar]
- Feng, G.D.; He, J.; Ao, X.; Chen, D.W. Effects of maize naturally contaminated with aflatoxin B1 on growth performance, intestinal morphology, and digestive physiology in ducks. Poult. Sci. 2017, 96, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.S.; Pillai, M.M. Effect of aflatoxin B1 in gastrointestine of mice. J. Ecophysiol. Occup. Health 2004, 4, 153–159. [Google Scholar]
- Fleming, S.E.; Youngman, L.D.; Ames, B.N. Intestinal cell proliferation is influenced by intakes of protein and energy, aflatoxin, and whole-body radiation. Nutr. Cancer 1994, 22, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Akinrinmade, F.J.; Akinrinde, A.S.; Amid, A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: Modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Res. 2016, 32, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]
- Moncada, D.M.; Kammanadiminti, S.J.; Chadee, K. Mucin and toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003, 19, 305–311. [Google Scholar] [CrossRef]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Andrianifahanana, M.; Moniaux, N.; Batra, S.K. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 2006, 1765, 189–222. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fang, J.; Peng, X.; Cui, H.; Zuo, Z.; Deng, J.; Chen, Z.; Lai, W.; Shu, G.; Tang, L. Effects of sodium selenite on aflatoxin B1-induced decrease of ileac T cell and the mRNA contents of IL-2, IL-6, and TNF-α in broilers. Biol. Trace Elem. Res. 2014, 159, 167–173. [Google Scholar] [CrossRef] [PubMed]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Delneste, Y.; Beauvillain, C.; Jeannin, P. Innate immunity: Structure and function of TLRs. Med. Sci. 2007, 23, 67–73. [Google Scholar] [CrossRef]
- Borrello, S.; Nicolò, C.; Delogu, G.; Pandolfi, F.; Ria, F. TLR2: A crossroads between infections and autoimmunity? Int. J. Immunopathol. Pharmacol. 2011, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Inoue, N.; Matsumoto, M.; Nomura, M.; Yamada, K.; Matsuda, Y.; Toyoshima, K.; Seya, T. Molecular cloning and functional characterization of chicken toll-like receptors. A single chicken toll covers multiple molecular patterns. J. Biol. Chem. 2001, 276, 47143–47149. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; El-Far, M.; Dupuy, F.P.; Abdel-Hakeem, M.S.; He, Z.; Procopio, F.A.; Shi, Y.; Haddad, E.K.; Ancuta, P.; Sekaly, R.P.; et al. HCV RNA Activates APCs via TLR7/TLR8 while virus selectively stimulates macrophages without inducing antiviral responses. Sci. Rep. 2016, 6, 29447. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.V. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 2000, 12, 13–19. [Google Scholar] [CrossRef]
- Thoma-Uszynski, S.; Stenger, S.; Takeuchi, O.; Ochoa, M.T.; Engele, M.; Sieling, P.A.; Barnes, P.F.; Rollinghoff, M.; Bolcskei, P.L.; Wagner, M.; et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 2001, 291, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirement of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994; ISBN 978-0-309-04892-7. [Google Scholar]
- Kaoud, H.A. Innovative methods for the amelioration of aflatoxin (AFB1) effect in broiler chicks. Spec. J. Biol. Sci. 2015, 1, 19–24. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2012, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

 ), G: Goblet cells, LY: lysosomes (□), N: nucleus, JC: Junctional complexes (→), TW: terminal web. Scale bar = 3 μm.
), G: Goblet cells, LY: lysosomes (□), N: nucleus, JC: Junctional complexes (→), TW: terminal web. Scale bar = 3 μm.
 ), G: Goblet cells, LY: lysosomes (□), N: nucleus, JC: Junctional complexes (→), TW: terminal web. Scale bar = 3 μm.
), G: Goblet cells, LY: lysosomes (□), N: nucleus, JC: Junctional complexes (→), TW: terminal web. Scale bar = 3 μm.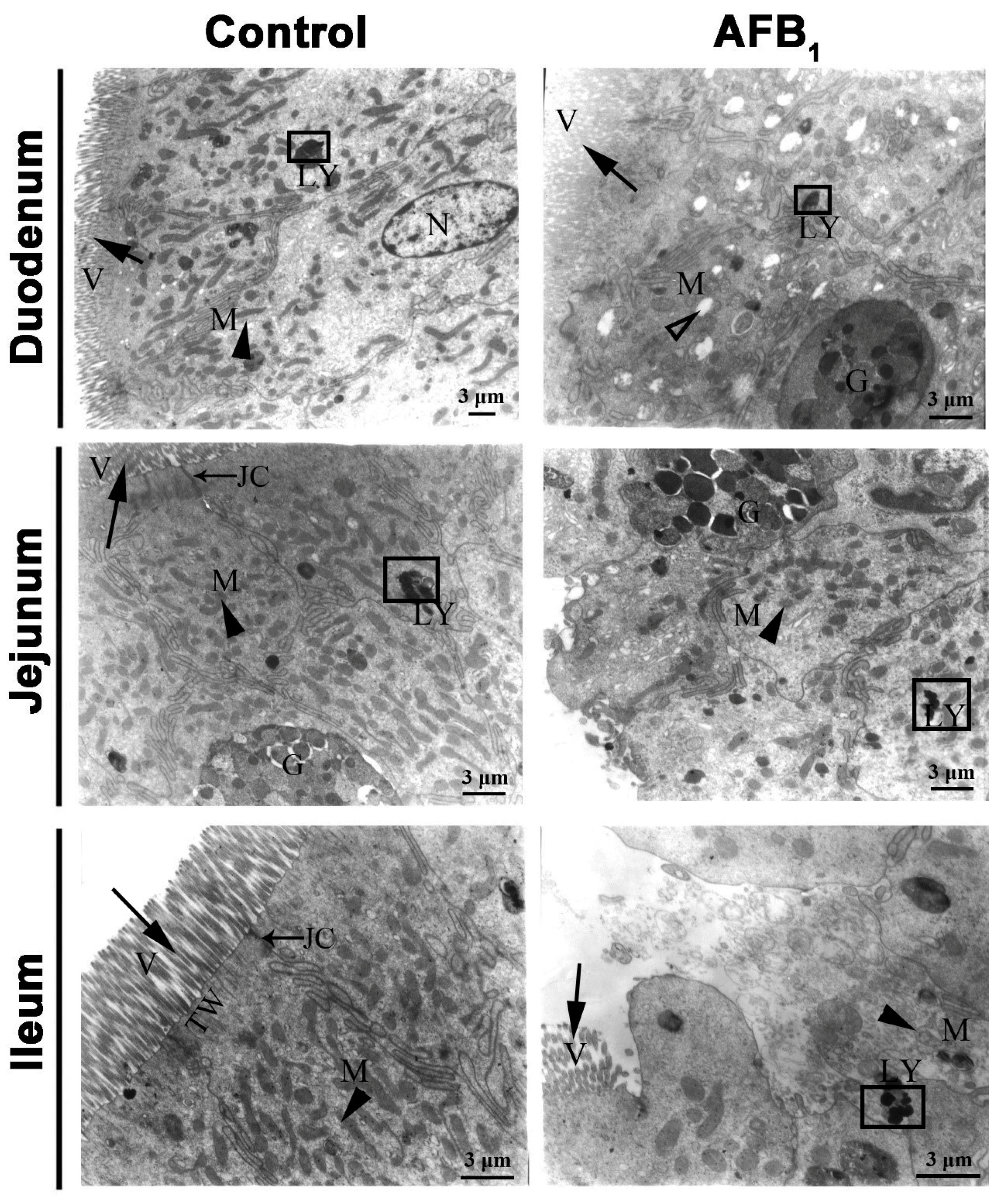
| Gene | Primer | Sequences (5′-3′) | Accession Number |
|---|---|---|---|
| TLR2-2 | F | CTGGGAAGTGGATTGTGGAC | AB046533.2 |
| R | CCAGCTCATACTTGCACCAC | ||
| TLR4 | F | AGCTACGAGGTTCTGCTCCA | AY064697 |
| R | TGTCCTGTGCATCTGAAAGC | ||
| TLR7 | F | TTATGCCACTCCTCTCTACCG | NM_001011688.2 |
| R | GCAGCCACCTCTGAAAGATT | ||
| β-actin | F | TGCTGTGTTCCCATCTATCG | L08165 |
| R | TTGGTGACAATACCGTGTTCA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zuo, Z.; Chen, K.; Gao, C.; Yang, Z.; Zhao, S.; Li, J.; Song, H.; Peng, X.; Fang, J.; et al. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1. Toxins 2018, 10, 131. https://doi.org/10.3390/toxins10040131
Wang F, Zuo Z, Chen K, Gao C, Yang Z, Zhao S, Li J, Song H, Peng X, Fang J, et al. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1. Toxins. 2018; 10(4):131. https://doi.org/10.3390/toxins10040131
Chicago/Turabian StyleWang, Fengyuan, Zhicai Zuo, Kejie Chen, Caixia Gao, Zhuangzhi Yang, Song Zhao, Jianzhen Li, Hetao Song, Xi Peng, Jing Fang, and et al. 2018. "Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1" Toxins 10, no. 4: 131. https://doi.org/10.3390/toxins10040131





