Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids
Abstract
:1. Introduction
2. Results and Discussion
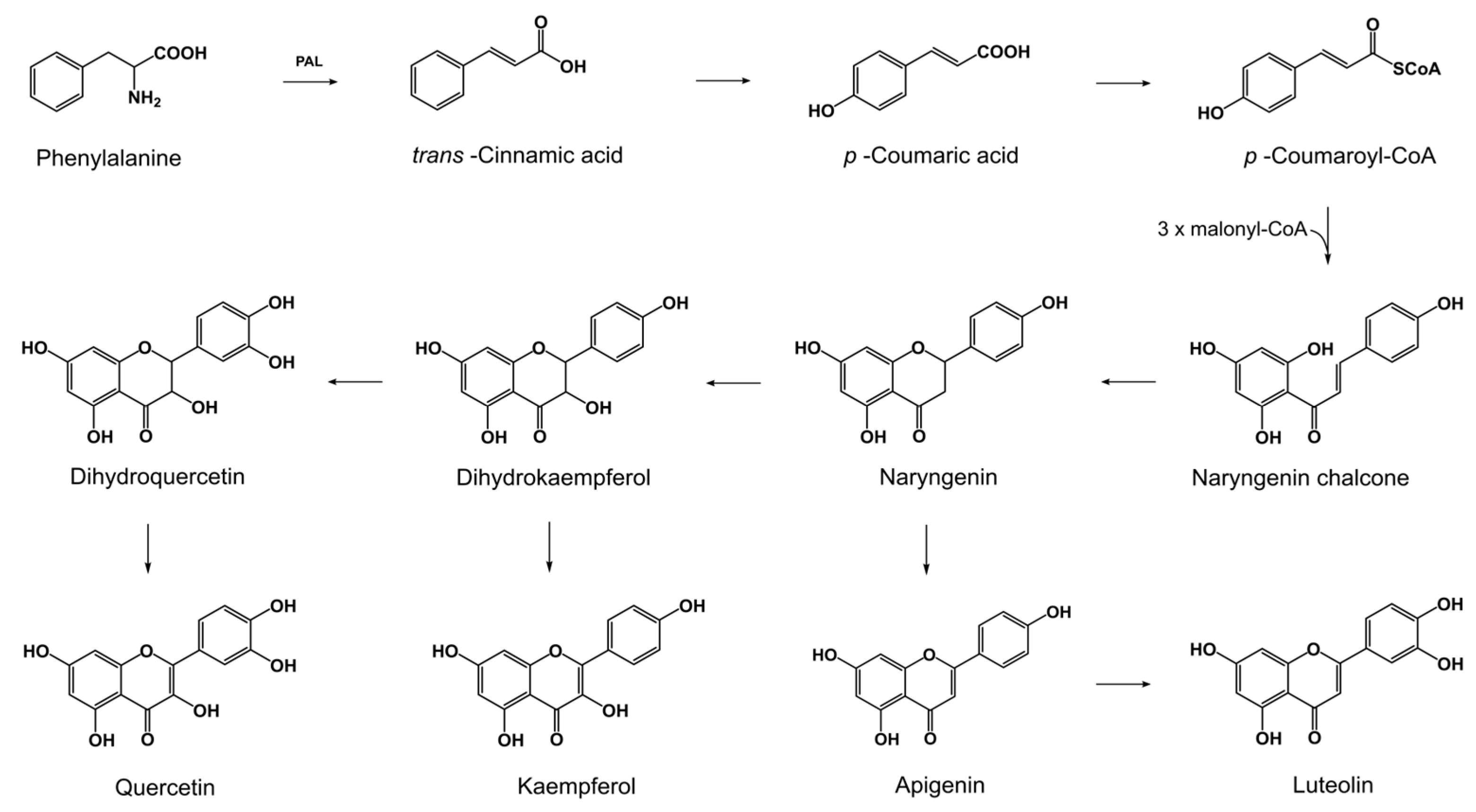
2.1. Production of Flavonoids by Fusaria
2.2. Reduction of Exogenous Flavonoids by Fusaria
2.3. Exogenous Flavonoids Affect Production of Phenolic Acids by Fusaria
2.4. Effect of Flavonoids on Trichothecene Accumulation in the Media
2.5. Effect of Flavonoids on the Expression of Tri Genes
3. Materials and Methods
3.1. Fungal Strains
3.2. Medium and Culture Conditions
3.3. Determination of Flavonoids and Phenolic Acids in the Medium
3.4. Determination of the Antioxidant Capacity (VCEAC/L) and Radical Scavenging Activity (ABTS+) of Flavonoids
3.5. Analysis of Trichothecenes from Fungal Cultures
3.6. Extraction of Total RNA and Preparation of cDNA
3.7. Gene Expression Analysis
3.8. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karlsson, I.; Friberg, H.; Kolseth, A.-K.; Steinberg, C.; Persson, P. Agricultural factors affecting Fusarium communities in wheat kernels. Int. J. Food Microbiol. 2017, 252, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E. Fusarium Mycotoxins Chemistry. Genetics and Biology; American Phytopathological Society Press: Saint Paul, MN, USA, 2006; ISBN 0-89-54-335-6. [Google Scholar]
- Trail, F. For Blighted Waves of Grain: Fusarium graminearum in the Postgenomics Era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á.; Tóth, B.; Varga, M.; Bartók, T.; Szabó-Hevér, Á.; Farády, L.; Lehoczki-Krsjak, S. Role of fungicides, application of nozzle types, and the resistance level of wheat varieties in the control of Fusarium head blight and deoxynivalenol. Toxins 2011, 3, 1453–1483. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Müller, M.D.; Poiger, T. Azole Fungicides: Occurrence and Fate in Wastewater and Surface Waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Sandstrom, M.W.; Kuivila, K.M.; Kolpin, D.W.; Meyer, M.T. Occurrence of azoxystrobin, propiconazole, and selected other fungicides in US streams, 2005–2006. Water Air Soil Pollut. 2011, 218, 307–322. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Tang, C.; Eriksson, E.; Jönsson, K.; Vollertsen, J.; Bester, K. Biocides in urban wastewater treatment plant influent at dry and wet weather: Concentrations, mass flows and possible sources. Water Res. 2014, 60, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; de Vega, C.; Pozo, M.I.; Lenaerts, M.; Van Assche, A.; Herrera, C.M.; Jacquemyn, H.; Lievens, B. Nectar yeasts of the Metschnikowia clade are highly susceptible to azole antifungals widely used in medicine and agriculture. FEMS Yeast Res. 2016, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the Development of Novel Strategies in Plant Disease Management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Pénichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to decipher the chemical defense of cereals against Fusarium graminearum and deoxynivalenol accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, B.; Ehlting, J.; Barbazuk, B.; Douglas, C.J. Comparative genomics of the shikimate pathway in Arabidopsis, Populus trichocarpa and Oryza sativa: Shikimate pathway gene family structure and identification of candidates for missing links in phenylalanine biosynthesis. In Recent Advances in Phytochemistry. Volume 40. Integrative Plant Biochemistry; Romeo, J.T., Ed.; Elsevier Ltd.: Oxford, UK, 2006; pp. 85–113. ISBN 9780080451251. [Google Scholar]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic Amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and Phenylalanine Biosynthesis in the Green Lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and Plant Phenylalanine Ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Joe, E.J.; Ahn, J.H. Molecular characterization of flavonol synthase from poplar and its application to the synthesis of 3-O-methylkaempferol. Biotechnol. Lett. 2010, 32, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, F.; Zeng, J.; Zhan, J. Type III polyketide synthases in natural product biosynthesis. IUBMB Life 2012, 64, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Seshime, Y.; Kitamoto, K. Genomics reveals traces of fungal phenylpropanoid-flavonoid metabolic pathway in the filamentous fungus Aspergillus oryzae. J. Microbiol. 2005, 43, 475–486. [Google Scholar] [PubMed]
- Seshime, Y.; Juvvadi, P.R.; Fujii, I.; Kitamoto, K. Discovery of a novel superfamily of type III polyketide synthases in Aspergillus oryzae. Biochem. Biophys. Res. Commun. 2005, 331, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, C.A.; Guldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Alegre, L.; van Breusegem, F.; Munne-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Padmavati, M.; Sakthivel, N.; Thara, K.V.; Reddy, A.R. Differential sensitivity of rice pathogens to growth inhibition by flavonoids. Phytochemistry 1997, 46, 499–502. [Google Scholar] [CrossRef]
- Parvez, M.M.; Tomita-Yokotani, K.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Weidenbörner, M.; Cavaleiro, J.A.S. Growth control of different Fusarium species by selected flavones and flavonoid mixtures. Mycol. Res. 1998, 102, 638–640. [Google Scholar] [CrossRef]
- Bollina, V.; Kumaraswamy, G.K.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Faubert, D.; Hamzehzarghani, H. Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Mol. Plant Pathol. 2010, 11, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kushalappa, A.C. In vitro inhibition of trichothecene biosynthesis in Fusarium graminearum by resistance-related endogenous metabolites identified in barley. Mycology 2011, 2, 291–296. [Google Scholar] [CrossRef]
- Chitarrini, G.; Nobili, C.; Pinzari, F.; Antonini, A.; de Rossi, P.; del Fiore, A.; Procacci, S.; Tolaini, V.; Scala, V.; Scarpari, M.; et al. Buckwheat achenes antioxidant profile modulates Aspergillus flavu growth and aflatoxin production. Int. J. Food Microbiol. 2014, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.A. Inhibition of aflatoxin B1 biosynthesis in Aspergillus flavus by anthocyanidins and related flavonoids. J. Agric. Food Chem. 1999, 47, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.P.; Reynoso, C.M.; Céliz, G.; Daz, M.; Resnik, S.L. Efficacy of flavanones obtained from citrus residues to prevent patulin contamination. Food Res. Int. 2012, 48, 930–934. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D.; Spencer, G.F. Inhibition of trichothecene toxin biosynthesis by naturally-occurring shikimate aromatics. Phytochemistry 1988, 27, 767–771. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Ochiai, N.; Tokai, T.; Ohsato, S.; Nishiuchi, T.; Yoshida, M.; Fujimura, M.; Kimura, M. A screening system for inhibitors of trichothecene biosynthesis: Hydroxylation of trichodiene as a target. Biotechnol. Lett. 2008, 30, 1055. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Identification of metabolites related to mechanisms of resistance in barley against Fusarium graminearum, based on mass spectrometry. Plant Mol. Biol. 2011, 77, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Gunnaiah, R.; Kushalappa, A.C.; Duggavathi, R.; Fox, S.; Somers, D.J. Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against Fusarium graminearum. PLoS ONE 2012, 7, e40695. [Google Scholar] [CrossRef] [PubMed]
- Buśko, M.; Góral, T.; Ostrowska, A.; Matysiak, A.; Walentyn-Góral, D.; Perkowski, J. The effect of Fusarium inoculation and fungicide application on concentrations of flavonoids (apigenin, kaempferol, luteolin, naringenin, quercetin, rutin, vitexin) in winter wheat cultivars. Am. J. Plant Sci. 2014, 5, 3727–3736. [Google Scholar] [CrossRef]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Sinapic Acid Affects Phenolic and Trichothecene Profiles of F. culmorum and F. graminearum Sensu Stricto. Toxins 2017, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Trans-Cinnamic and Chlorogenic Acids Affect the Secondary Metabolic Profiles and Ergosterol Biosynthesis by Fusarium culmorum and F. graminearum Sensu Stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wood, K.V.; Morgan, J.A. Metabolic Engineering of the Phenylpropanoid Pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.H. Biosynthesis of Two Flavones, Apigenin and Genkwanin in Escherichia Coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Gutiérrez-del-Río, I.; Yagüe, P.; Manteca, Á.; Villar, C.J.; Lombó, F. De Novo Biosynthesis of Apigenin, Luteolin, and Eriodictyol in the Actinomycete Streptomyces albus and Production Improvement by Feeding and Spore Conditioning. Front. Microbiol. 2017, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.; Yan, Y.; Koffas, M.A.G. Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab. Eng. 2006, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, S.G.; Siedler, S.; Malla, S.; Harrison, S.J.; Maury, J.; Neves, A.R.; Forster, J. Assembly of a novel biosynthetic pathway for production of the plant flavonoid fisetin in Escherichia coli. Metab. Eng. 2015, 31, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-G.; Liu, B.-G.; Liang, G.-Z.; Ning, Z.-X. Structure-Activity Relationship of Flavonoids Active against Lard Oil Oxidation Based on Quantum Chemical Analysis. Molecules 2009, 14, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, S.-H.; Shin, J.Y.; Kim, H.-K.; Yun, S.-H.; Kim, H.-Y.; Ryu, J.-G. Comparison of Trichothecene Biosynthetic Gene Expression between Fusarium graminearum and Fusarium asiaticum. Plant Pathol. J. 2014, 30, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, R.; Sun, X.; Li, G.; Yang, Q.; Li, Q.; Gai, W.; Zhang, M.; Chen, L.; Yang, G.; et al. The effect of the skeleton structure of flavanone and flavonoid on interaction with transferrin. Bioorg. Med. Chem. Lett. 2013, 23, 6677–6681. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Tag, A.G.; Garifullina, G.F.; Peplow, A.W.; Ake, C., Jr.; Phillips, T.D.; Hohn, T.M.; Beremand, M.N. A novel regulatory gene, Tri10, controls trichothecene toxin production and gene expression. Appl. Environ. Microbiol. 2001, 67, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- Markovic, J.M.D.; Milenkovic, D.; Amie, D.; Popovic-Bijelic, A.; Mojovic, M.; Pasti, I.A.; Markovic, Z.S. Energy requirements of the reactions of kaempferol and selected radical species in different media: Towards the prediction of the possible radical scavenging mechanisms. Struct. Chem. 2014, 2, 1795–1804. [Google Scholar] [CrossRef]
- Kulik, T.; Buśko, M.; Pszczółkowska, A.; Perkowski, J.; Okorski, A. Plant lignans inhibit growth and trichothecene biosynthesis in Fusarium graminearum. Lett. Appl. Microbiol. 2014, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lorenc-Kukuła, K.; Wróbel-Kwiatkowska, M.; Starzycki, M.; Szopa, J. Engineering flax with increased flavonoid content and thus Fusarium resistance. Physiol. Mol. Plant Pathol. 2007, 70, 38–48. [Google Scholar] [CrossRef]
- Gill, U.S.; Uppalapati, S.R.; Gallego-Giraldo, L.; Ishiga, Y.; Dixon, R.A.; Mysore, K.S. Metabolic flux towards the (iso)flavonoid pathway in lignin modified alfalfa lines induces resistance against Fusarium oxysporum f. sp. medicaginis. Plant Cell Environ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Abarenkov, K.; Buśko, M.; Bilska, K.; van Diepeningen, A.D.; Ostrowska-Kołodziejczak, A.; Krawczyk, K.; Brankovics, B.; Stenglein, S.; Sawicki, J.; et al. ToxGen: An improved reference database for the identification of type B-trichothecene genotypes in Fusarium. PeerJ 2017, 5, e2992. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.L.; Barreau, C.; Richard-Forget, F. Cinnamic-derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellergini, N.; Proteggente, A.; Pannala, A.S.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Perkowski, J.; Kiecana, I.; Kaczmarek, Z. Natural occurrence and distribution of Fusarium toxins in contaminated barley cultivars. Eur. J. Plant Pathol. 2003, 109, 331–339. [Google Scholar] [CrossRef]
- Kulik, T.; Łojko, M.; Jestoi, M.; Perkowski, J. Sublethal concentrations of azoles induce tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012, 335, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
| Subclass | Compound | Substitution at Carbon Position | Double Bond C2-C3 | Antioxidant Activity | Antiradical Activity 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 5 | 7 | 3′ | 4′ | VCEAC/L | ABTS (μM TROLOX/100 g) | Protection Factor 1 | (%) | |||
| Flavones | Apigenin | - | OH | OH | - | OH | + | 190.4 | 297.5 | 0.99 | 0.7 |
| Luteolin | - | OH | OH | OH | OH | + | 483.5 | 589.7 | 4.24 | n.t. | |
| Flavonols | Kaempferol | OH | OH | OH | - | OH | + | 450.7 | 540.3 | 2.49 | 93.5 |
| Quercetin | OH | OH | OH | OH | OH | + | 692.5 | 744.9 | 11.50 | 89.8 | |
| Flavanones | Naringenin | - | OH | OH | - | OH | - | 301.7 | 529.4 | 1.09 | 6.3 |
| Species | Strain | Trichothecene Genotype | Origin, Host and Year of Isolation |
|---|---|---|---|
| F. culmorum | CBS 173.31, NRRL 26853 | 3ADON | Canada, oat, 1927 |
| MUCL 53469 | 3ADON | Belgium, corn, 2007 | |
| CBS 139512 | NIV | Poland, wheat, 2003 | |
| F. graminearum s.s. | CBS 119173, NRRL 38369 | 3ADON | USA, Louisiana, wheat, 2005 |
| CBS 138561 | 15ADON | Poland, wheat, 2010 | |
| MUCL 53455 | NIV | Belgium, corn, 2007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. https://doi.org/10.3390/toxins10030110
Bilska K, Stuper-Szablewska K, Kulik T, Buśko M, Załuski D, Jurczak S, Perkowski J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins. 2018; 10(3):110. https://doi.org/10.3390/toxins10030110
Chicago/Turabian StyleBilska, Katarzyna, Kinga Stuper-Szablewska, Tomasz Kulik, Maciej Buśko, Dariusz Załuski, Sebastian Jurczak, and Juliusz Perkowski. 2018. "Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids" Toxins 10, no. 3: 110. https://doi.org/10.3390/toxins10030110





