Human Scalp Hair as an Indicator of Exposure to the Environmental Toxin β-N-Methylamino-l-alanine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Association of BMAA to Human Hair
2.2. BMAA in Human Scalp Hair
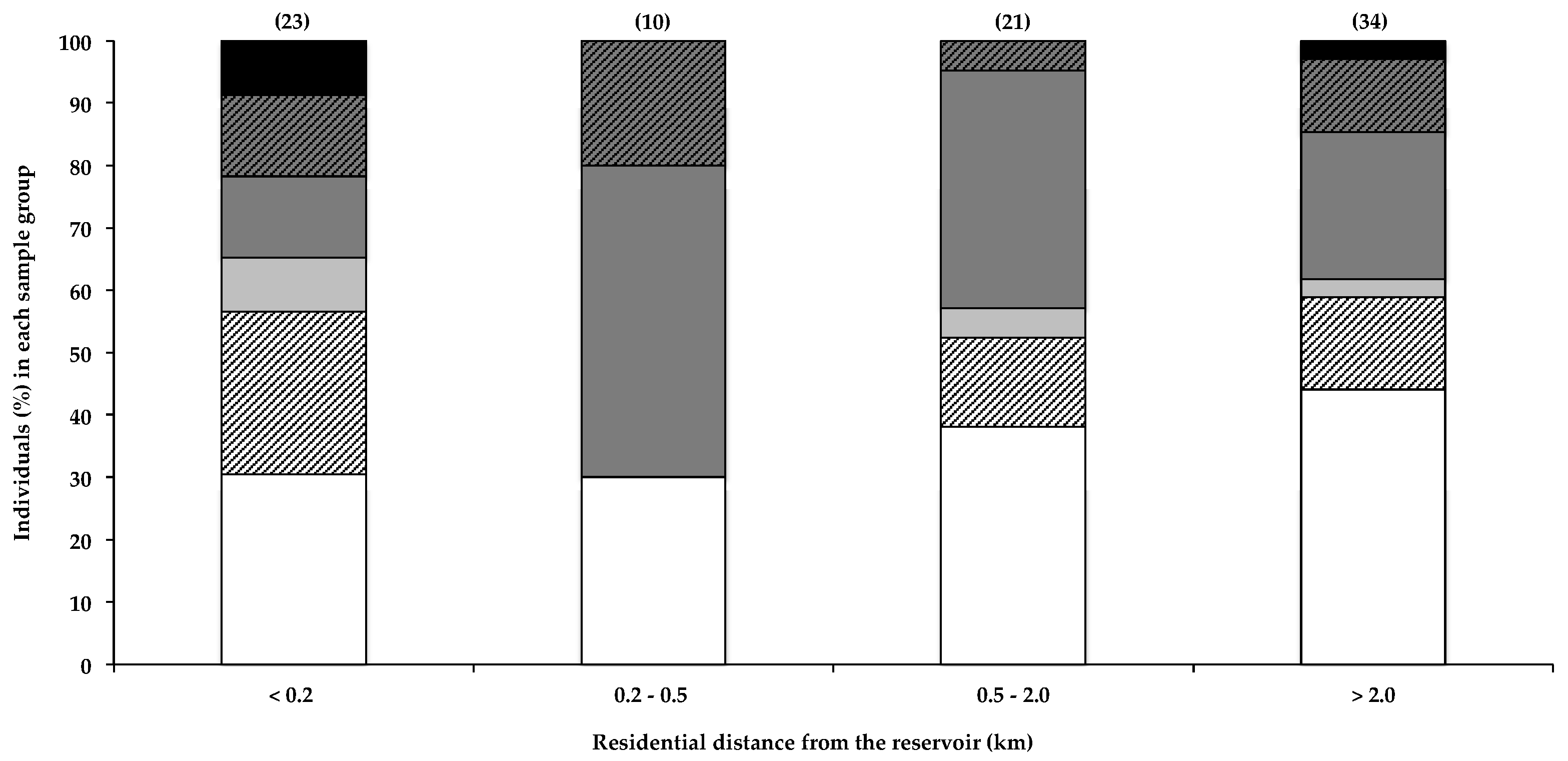
2.2.1. Residential Proximity to the Water
2.2.2. Frequency of Reservoir-Based Water Sports
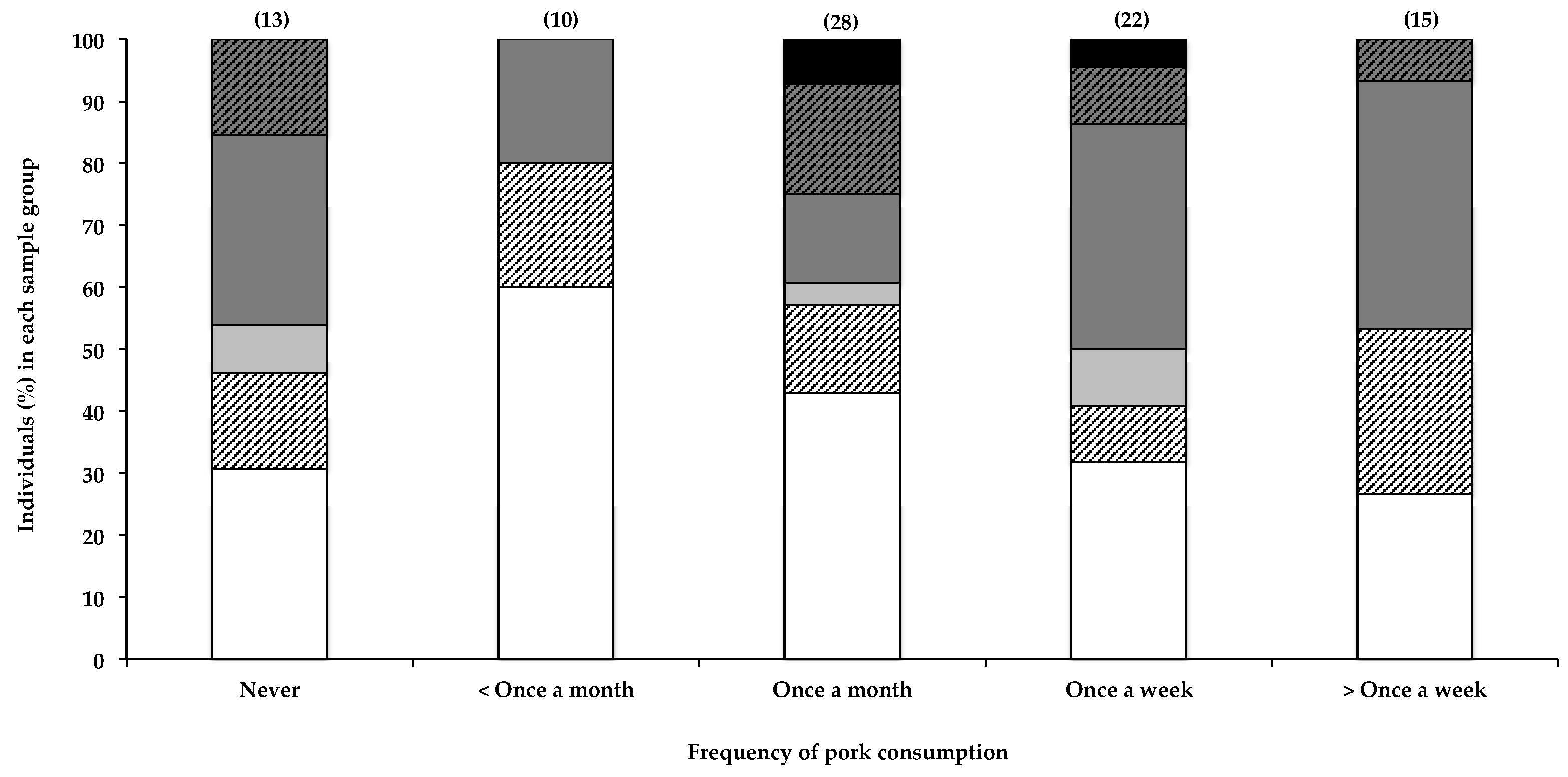
2.2.3. Consumption of Fish Sourced from the Reservoir
2.2.4. Consumption of Shellfish and Other Dietary Items
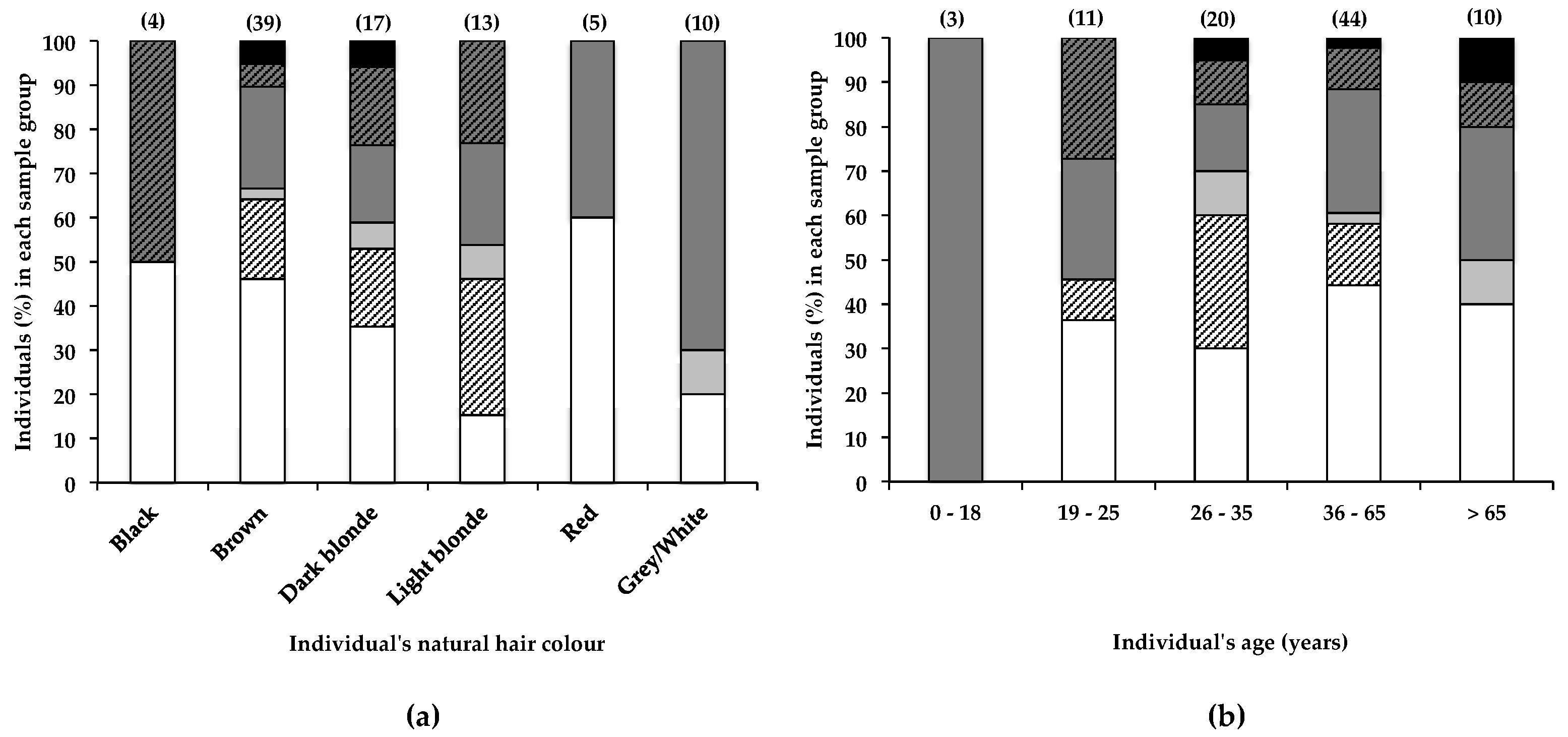
2.3. Other Factors to Consider
3. Conclusions
4. Materials and Methods
4.1. Study Site
4.2. Hair Samples and Questionnaires
4.2.1. Ethical Statement
4.2.2. Questionnaires
4.2.3. Hair Sample Collection
4.3. Hair Sample Analysis
4.3.1. Hair Sample Pre-Treatment
4.3.2. BMAA Analysis
4.3.3. Surface Binding of BMAA
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trojsi, F.; Monsurrò, M.R.; Tedeschi, G. Exposure to Environmental Toxicants and Pathogenesis of Amyotrophic Lateral Sclerosis: State of the Art and Research Perspectives. Int. J. Mol. Sci. 2013, 14, 15286–15311. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Calne, D. Amyotrophic Lateral Sclerosis, Parkinson’s Disease and Alzheimer’s Disease: Phylogenetic Disorders of the Human Neocortex Sharing Many Characteristics. Can. J. Neurol. Sci. 1992, 19, 117–120. [Google Scholar] [PubMed]
- Ingre, C.; Roos, P.M.; Piehl, F.; Kamel, F.; Fang, F. Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 2015, 7, 181–193. [Google Scholar] [PubMed]
- Bradley, W.G.; Miller, R.X.; Levine, T.D.; Stommel, E.W.; Cox, P.A. Studies of Environmental Risk Factors in Amyotrophic Lateral Sclerosis (ALS) and a Phase I Clinical Trial of l-Serine. Neurotox. Res. 2017, 33, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yegambaram, M.; Manivannan, B.; Beach, T.G.; Halden, R.U. Role of Environmental Contaminants in the Etiology of Alzheimer’s Disease: A Review. Curr. Alzheimer Res. 2015, 12, 116–146. [Google Scholar] [CrossRef] [PubMed]
- Betemps, E.J.; Buncher, R.C. Birthplace as a Risk Factor in Motor Neurone Disease and Parkinson’s Disease. Int. J. Epidemiol. 1993, 22, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Banack, S.A.; Metcalf, J.S.; Cox, P.A.; Downing, T.G. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-l-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for neurotoxin BMAA in aquatic environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef] [PubMed]
- Reveillon, D.; Sechet, V.; Hess, P.; Amzil, Z. Production of BMAA and DAB by diatoms (Phaeodactylum tricornutum, Chaetoceros sp., Chaetoceros calcitrans and Thalassiosira pseudonana) and bacteria isolated from a diatom culture. Harmful Algae 2016, 58, 45–50. [Google Scholar] [PubMed]
- Lage, S.; Costa, P.R.; Moita, T.; Eriksson, J.; Rasmussen, U.; Rydberg, S.J. BMAA in shellfish from two Portuguese transitional water bodies suggests the marine dinoflagellate Gymnodinium catenatum as a potential BMAA source. Aquat. Toxicol. 2014, 152, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Downing, T.G. Animal Model of Neurodegenerative Disease. Provisional Patent Application No. 1705535.1, 5 April 2017. [Google Scholar]
- Brand, L.E. Human exposure to cyanobacteria and BMAA. Amyotroph. Lateral Scler 2009, 10, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin β-N-methylamino-l-alanine (BMAA) in south Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spacil, Z.; Ilag, L.L.; Ronnevi, L.O.; Rasmusse, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef]
- Jiang, L.; Kiselova, N.; Rosén, J.; Ilag, L.L. Quantification of neurotoxin BMAA (β-N-methylamino-l-alanine) in seafood from Swedish markets. Sci. Rep. 2014, 4, 6931. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Metcalf, J.S.; Bradley, W.G.; Cox, P.A. Detection of cyanobacterial neurotoxin β-N-methylamino-l-alanine within shellfish in the diet of an ALS patient in Florida. Toxicon 2014, 90, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Reveillon, D.; Abadie, E.; Sechet, V.; Brient, L.; Savar, V.; Bardouil, M.; Hess, P.; Amzil, Z. Beta-N-methylamino-l-alanine: LC-MS/MS optimization, screening of cyanobacterial strains and occurrence in shellfish from Thau, a French Mediterranean lagoon. Mar. Drugs 2014, 12, 5441–5467. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Q.; Chen, X.; Wang, X.; Liao, X.; Jiang, L.; Wu, J.; Yang, L. Occurrence and transfer of a cyanobacterial neurotoxin β-methylamino-l-alanine within the aquatic food webs of Gonghu Bay (Lake Taihu, China) to evaluate the potential human health risk. Sci. Total Environ. 2014, 468–469, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Caller, T.A.; Field, N.C.; Chipman, J.W.; Shi, X.; Harris, B.T.; Stommel, E.W. Spatial clustering of Amyotrophic lateral sclerosis and the potential role of BMAA. Amyotroph. Lateral Scler. 2012, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Stommel, E.W.; Field, N.C.; Caller, T.A. Aerosolization of cyanobacteria as a risk factor for amyotrophic lateral sclerosis. Med. Hypotheses 2013, 80, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.L.; Downing, S.; Downing, T.G. The Evaluation of BMAA Inhalation as a Potential Exposure Route Using a rat Model. Neurotox. Res. 2017, 33, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of Cyanotoxins, β-N-methylamino-l-alanine and Microcystins, from a Lake Surrounded by Cases of Amyotrophic Lateral Sclerosis. Toxins 2015, 7, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Banack, S.A.; Richer, R.; Cox, P.A. Neurotoxic amino acids and their isomers in desert environments. J. Arid Environ. 2015, 112, 140–144. [Google Scholar] [CrossRef]
- Richer, R.; Banack, S.A.; Metcalf, J.S.; Cox, P.A. The persistence of cyanobacterial toxins in desert soils. J. Arid Environ. 2015, 112, 134–139. [Google Scholar] [CrossRef]
- Caller, T.A.; Doolin, J.W.; Haney, J.F.; Murby, A.J.; West, K.G.; Farrar, H.E.; Ball, A.; Harris, B.T.; Stommel, E.W. A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms. Amyotroph. Lateral Scler. 2009, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Berntzon, L.; Ronnevi, L.O.; Bergman, B.; Eriksson, J. Detection of BMAA in the human central nervous system. Neuroscience 2015, 292, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, N.; Hill, D.J.; Diggs, D.L.; Faison, B.D.; Francis, B.M.; Lang, J.R.; Larue, M.M.; Le, T.-T.; Loftin, K.A.; Lugo, J.N.; et al. A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-l-alanine, in neurodegenerative disease in humans. J. Toxicol. Environ. Health Part B 2017, 20, 183–229. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Cox, P.A.; Murch, S.J. Flying Fox Consumption and Human Neurodegenerative Disease in Guam. In Island Bats: Evolution, Ecology, and Conservation, 1st ed.; Fleming, T.H., Racey, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 341–366. [Google Scholar]
- Banack, S.A.; Cox, P.A. Biomagnification of cycad neurotoxins in flying foxes: Implications for ALS/PDC in Guam. Neurology 2013, 61, 387–389. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Kotut, K.; Krienitz, L.; Codd, G.A. Amino acid neurotoxins in feathers of the Lesser Flamingo, Phoeniconaias minor. Chemosphere 2013, 90, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Cox, P.A. Creating a Simian Model of Guam ALS/PDC Which Reflects Chamorro Lifetime BMAA Exposures. Neurotox. Res. 2017, 33, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.L. Mechanisms of drug incorporation into hair. Forens. Sci. Int. 1993, 63, 19–29. [Google Scholar] [CrossRef]
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Boumba, V.A.; Ziavrou, K.S.; Vougiouklakis, T. Hair as a Biological Indicator of Drug Use, Drug Abuse or Chronic Exposure to Environmental Toxicants. Int. J. Toxicol. 2006, 25, 143–163. [Google Scholar] [CrossRef] [PubMed]
- De Laudera, S.F.; Kidwell, D.A. The incorporation of dyes into hair as a model for drug binding. Forensic Sci. Int. 2000, 107, 93–104. [Google Scholar] [CrossRef]
- Ashton, P.J.; Chutter, P.M.; Cochrane, K.L.; De Moor, F.C.; Hely-Hutchinson, J.R.; Jarvis, A.C.; Robarts, R.D.; Scott, W.E.; Thornton, J.A.; Twinch, A.J.; et al. The Limnology of the Hartbeespoort Dam; South African National Scientific Programmes Report No. 110; National Scientific Programmes Unit: Pretoria, South Africa, 1985; p. 269. [Google Scholar]
- Scott, L.L.; Downing, S.; Phelan, R.R.; Downing, T.G. Environmental modulation of microcystin and β-N-methylamino-l-alanine as a function of nitrogen availability. Toxicon 2014, 87, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Massetet, E.; Banack, S.; Boumediene, F.; Abadie, E.; Brient, L.; Pernet, F.; Juntas-Morales, R.; Pageot, N.; Metcalf, J.; Cox, P.; et al. Dietary BMAA exposure in an ALS cluster from southern France. PLoS ONE 2014, 8, e83406. [Google Scholar]
- Corcia, P.; Jafari-Schluep, H.F.; Lardillier, D.; Mazyad, H.; Giraud, P.; Clavelou, P.; Pouget, J.; Camu, W. A clustering of conjugal ALS in southeastern France. Arch. Neurol. 2003, 60, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Contardo-Jara, V.; Schwanemann, T.; Pflugmacher, S. Uptake of a cyanotoxin, β-N-methylamino-l-alanine, by wheat (Triticum aestivum). Ecotoxicol. Environ. Saf. 2014, 104, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kronstrand, R.; Förstberg-Peterson, S.; KÅgedal, B.; Ahlner, J.; Larson, G. Codeine Concentration in Hair after Oral Administration Is Dependent on Melanin Content. Clin. Chem. 1999, 45, 1485–1494. [Google Scholar] [PubMed]
- Karlsson, O.; Berg, C.; Brittebo, E.B.; Lindquist, N.G. Retention of the cyanobacterial neurotoxin β-N-methylamino-l-alanine in melanin and neuromelanin-containing cells—A possible link between Parkinson-dementia complex and pigmentary retinopathy. Pigment Cell Melanoma Res. 2009, 22, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.W.; Bernard, S. Eutrophication and cyanobacteria in South Africa’s standing water bodies: A view from space. S. Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef]
- Boonzaaier, J. The development of a reconciliation strategy for the crocodile (west) water supply system. In Summary of Previous and Current Studies; Department of Water Affairs and Forestry: Pretoria, South Africa, 2008. [Google Scholar]
- Scott, L.L.; Downing, S.; Downing, T.G. Potential for dietary exposure to β-N-methylamino-l-alanine and Microcystin from a freshwater system. Toxicon 2018, in press. [Google Scholar]
- Mecolini, L.; Mandrioli, R.; Protti, M.; Contic, M.; Serpelloni, G.; Raggi, M.A. Monitoring of chronic Cannabis abuse: An LC–MS/MS method for hair analysis. J. Pharm. Biomed. Anal. 2013, 76, 119–125. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Schwartz, Z.; Jackson, G.P. δ13C analysis of amino acids in human hair using trimethylsilyl derivatives and gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; pp. 77–85. [Google Scholar]

| Hair BMAA Content (µg g−1) | Gender | Age | Ethnicity | Hair BMAA Content (µg g−1) | Gender | Age | Ethnicity |
|---|---|---|---|---|---|---|---|
| ND | F | 47 | C | ND | F | 25 | C |
| ND | F | 72 | C | ND | F | 63 | C |
| ND | F | 63 | C | ND | F | 58 | C |
| ND | F | 40 | C | ND | F | 55 | C |
| ND | F | 85 | C | ND | F | 62 | C |
| ND | F | 55 | C | ND | F | 48 | C |
| ND | F | 33 | C | ND | F | 20 | C |
| ND | F | 37 | C | ND | F | 24 | C |
| ND | F | 75 | C | ND | F | 33 | C |
| ND | F | 60 | C | ND | F | 43 | C |
| ND | F | 40 | C | ND | F | 47 | C |
| ND | F | 58 | C | ND | F | 51 | C |
| ND | F | 28 | C | ND | F | 72 | C |
| ND | F | 35 | C | ND | F | 34 | C |
| ND | F | 61 | C | ND | F | 44 | C |
| ND | M | 48 | C | 0.021 | F | 26 | C |
| ND | F | 25 | C | 0.025 | M | 23 | C |
| ND | F | 33 | C | 0.028 | F | 83 | C |
| <LOQ | F | 25 | C | 0.032 | M | 25 | C |
| <LOQ | F | 50 | C | 0.033 | F | 45 | C |
| <LOQ | F | 44 | C | 0.035 | F | 12 | C |
| <LOQ | F | 50 | C | 0.045 | F | 26 | C |
| <LOQ | F | 45 | C | 0.051 | F | 54 | C |
| <LOQ | F | 26 | C | 0.064 | F | 21 | C |
| <LOQ | F | 34 | C | 0.077 | F | 49 | C |
| <LOQ | F | 31 | C | 0.080 | M | 58 | C |
| <LOQ | F | 32 | C | 0.081 | F | 52 | C |
| <LOQ | F | 45 | C | 0.084 | F | 73 | C |
| <LOQ | F | 35 | C | 0.088 | F | 62 | C |
| <LOQ | F | 45 | C | 0.092 | F | 77 | C |
| <LOQ | F | 63 | C | 0.098 | M | 3 | C |
| <LOQ | M | 26 | C | 0.107 | F | 22 | A |
| 0.006 | F | 48 | C | 0.112 | F | 31 | A |
| 0.007 | M | 79 | C | 0.112 | F | 21 | C |
| 0.008 | F | 34 | C | 0.128 | F | 27 | C |
| 0.009 | F | 27 | C | 0.149 | F | 63 | C |
| 0.012 | M | 50 | C | 0.226 | F | 54 | C |
| 0.014 | F | 50 | C | 0.240 | F | 69 | C |
| 0.015 | M | 18 | C | 0.695 | F | 36 | C |
| 0.016 | F | 26 | C | 0.723 | F | 24 | C |
| 0.016 | F | 43 | C | 0.741 | F | 43 | C |
| 0.016 | F | 52 | C | 1.164 | M | 67 | C |
| 0.018 | F | 61 | C | 1.652 | F | 35 | C |
| 0.021 | F | 47 | C | 9.636 | F | 54 | C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Downing, S.; Scott, L.L.; Zguna, N.; Downing, T.G. Human Scalp Hair as an Indicator of Exposure to the Environmental Toxin β-N-Methylamino-l-alanine. Toxins 2018, 10, 14. https://doi.org/10.3390/toxins10010014
Downing S, Scott LL, Zguna N, Downing TG. Human Scalp Hair as an Indicator of Exposure to the Environmental Toxin β-N-Methylamino-l-alanine. Toxins. 2018; 10(1):14. https://doi.org/10.3390/toxins10010014
Chicago/Turabian StyleDowning, Simoné, Laura Louise Scott, Nadezda Zguna, and Timothy Grant Downing. 2018. "Human Scalp Hair as an Indicator of Exposure to the Environmental Toxin β-N-Methylamino-l-alanine" Toxins 10, no. 1: 14. https://doi.org/10.3390/toxins10010014




