1. Introduction
The role of dietary protein on increasing satiation, satiety, and energy metabolism is well documented [
1]. However, eating behavior is modulated by not just meal or diet macronutrient composition, but also the interactions among physiological need (homeostatic), the rewarding properties of food, and our environments [
2]. Changes to our food environment, coupled with the increase in adiposity over the last four decades, suggest that eating behavior is influenced more by nonhomeostatic contributors than homeostatic regulatory mechanisms. With the seemingly constant availability of energy-dense, highly palatable (enjoyable and appetizing) foods, understanding eating behavior beyond the physical need has become a priority [
2]. One factor that influences eating behavior is the reinforcing value of the foods available in an environment [
3]. Moreover, food reinforcement is positively associated with obesity [
4]. The reinforcing value of a food is a learned response that is associated with that food’s reinforcement history (positive or negative) and has been shown to be modifiable depending upon baseline intake and the availability of the alternative choices [
4,
5]. Therefore, elucidating the motivating properties of food is essential in developing successful strategies that work to enhance homeostatic regulation of food intake.
Dietary protein has been posited to influence food reinforcement regulatory mechanisms. Data from animal and human magnetic resonance imaging (MRI) studies demonstrate that greater protein intake diminishes activation of central motivation and reward areas of the brain [
6]. In animals, intragastric infusion of physiological amounts of protein decreased stimulation in the amygdala compared to a mixed nutrient meal or intravenous glucose infusion [
7]. In humans, greater dietary protein reduces activation in the mesocorticolimbic circuitry (notably the insular cortex, prefrontal cortex, hippocampus, and parahippocampus) [
8,
9,
10]. Collectively, these results demonstrate the potential role of dietary protein in modulating food reinforcement. However, it is not clear if reduced activity in these reward areas of the brain translates into actual decreases in reward-driven eating behaviors.
Food reinforcement has been studied using a computer-based choice task (operant responding) in a variety of settings and is measured by the amount of operant responding (i.e., work) an individual will complete to earn a small portion of the food [
3]. If the food is highly reinforcing, it will support more work to earn access to that food [
4]. The present study extends previous research by employing such an operant responding task to test the effects of increased dietary protein on actual motivated behavior and the reinforcing value of foods with specific taste profiles in healthy weight adults. We hypothesized that increasing dietary protein would decrease the reinforcing value for energy-dense, highly palatable snack foods.
3. Results
3.1. Reinforcing Value of Snack Foods
A main effect of gender (
F(1,19) = 4.49,
p < 0.05) and snack food type (
F(1,53) = 5.78,
p < 0.02) was found for P
response. Operant responding (P
response) for snack food was greater in the men (250 (167, 374) mouse clicks) compared to the women (75 (50, 111) mouse clicks) and for the sweet (194 (141, 266) mouse clicks) compared to the savory (97 (71, 133) mouse clicks) snack foods. In addition, there was a significant interaction between gender and snack food type,
F(1,53) = 17.75,
p < 0.0001 (
Figure 1a). The women’s P
response was greater for the sweet (195 (125, 303) mouse clicks) compared to the savory (29 (18, 44) mouse clicks) snack foods (
p = 0.0001). For the men, there was no difference in P
response for the sweet (193 (123, 302) mouse clicks) and savory (324 (206, 508) mouse clicks) snack foods. There was also a significant interaction between gender and dietary protein level,
F(1,53) = 4.69,
p = 0.03 (
Figure 1b). When the meal contained 15% E protein there was no difference in P
response between the women (117 (76, 180) mouse clicks) and men (206 (131, 323) mouse clicks). However, when the meal contained 30% E protein the women’s P
response was markedly less (
p < 0.03) than that of the men (48 (30, 76) and 303 (193, 475) mouse clicks, respectively).
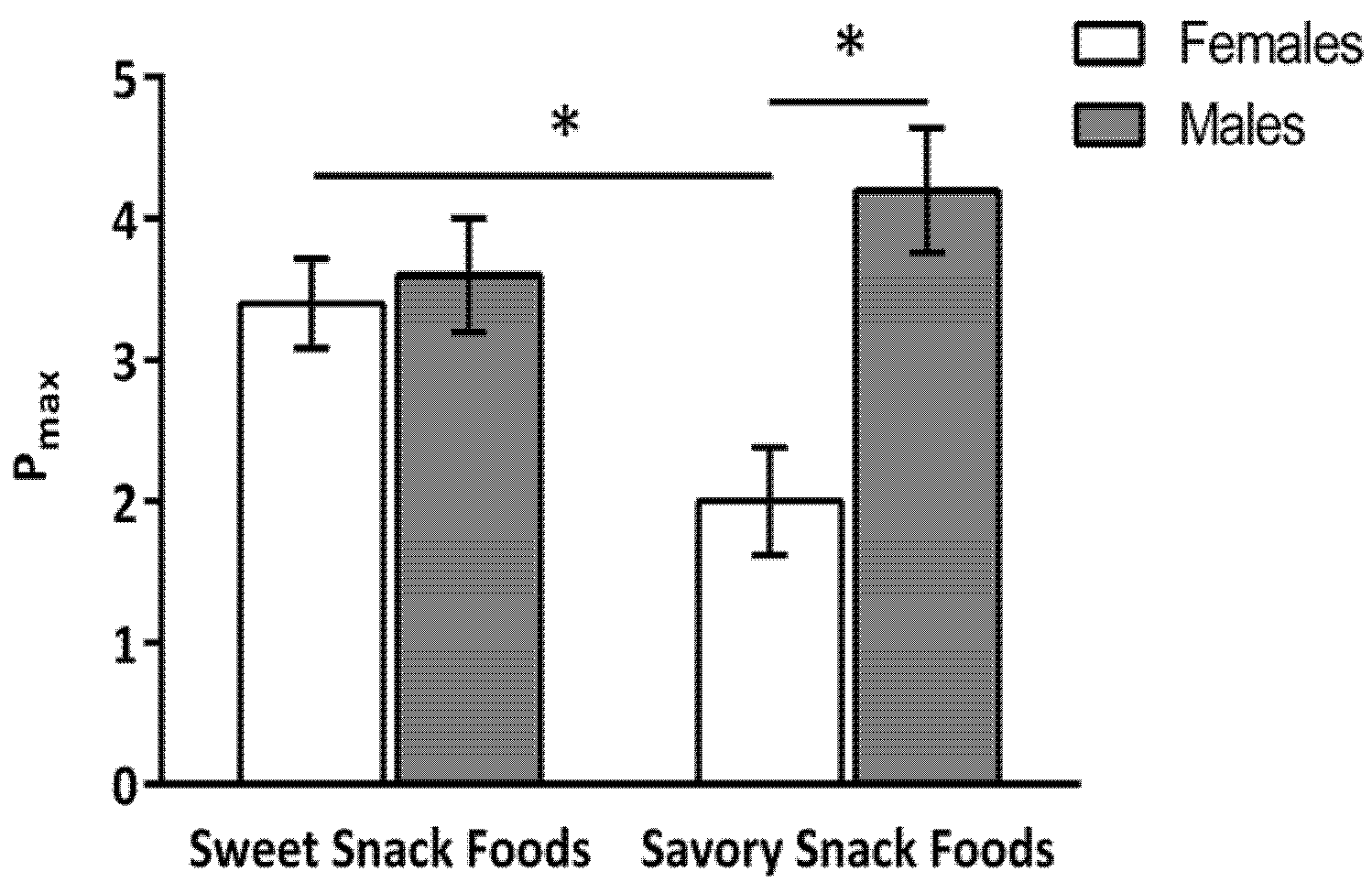
No significant main effect of gender, snack food type, or dietary protein level was found for P
max. However, there was a significant interaction between gender and snack food type,
F(1,53) = 14.68,
p < 0.01 (
Figure 2). For the women, P
max was greater for the sweet (3.4 ± 0.3 schedules) compared to the savory snack foods (2.0 ± 0.4 schedules). For the men, there was no difference in the P
max for the sweet (3.6 ± 0.4 schedules) and savory (4.2 ± 0.4 schedules) snack foods. Additionally, P
max for the savory snack foods was greater in the men than the women (
p < 0.02). There was no difference in P
max between the women and men for the sweet snack foods.
A main effect of gender (
F(1,18) = 13.17,
p < 0.01) and dietary protein level (
F(1,17) = 4.48,
p = 0.05) was found for RRV
sugar (
Figure 3). The interaction between gender and dietary protein level did not reach significance,
F(1,17) = 3.48,
p = 0.08. For the women, when the meal contained 15% protein the RRV
sugar was 0.59 ± 0.05 and when the meal contained 30% E protein RRV
sugar increased to 0.78 ± 0.09. This increase in RRV
sugar was driven by a decrease in the reinforcing value of the savory snack food (see
Figure 2). For the men, when the meal contained 15% E protein, RRV
sugar was 0.45 ± 0.03 and when the meal contained 30% protein the RRV
sugar was 0.47 ± 0.03.
3.2. Appetite Sensations
Main effects of gender and dietary protein level were found for hunger (F(1,30) = −16.40, p < 0.01 and F(1,18) = 5.18, p = 0.04, respectively) and fullness (F(1,30) = 12.79, p = 0.01 and F(1,18) = 5.54, p = 0.03, respectively). Women reported feeling less hungry and fuller than the men. Increasing dietary protein decreased feelings of hunger and increased feelings of fullness. There was no interaction between gender and dietary protein level.
A main effect of gender was found for the desire to eat something savory (F(1,35) = 13.91, p < 0.01). The desire to eat something savory was less in the women than the men irrespective of dietary protein level. The desire to eat something sweet did not differ between the women and men and was not influenced by dietary protein level.
3.3. Snack Food Intake
A main effect of gender was found for the amount of savory snack foods consumed, F(1,19) = 10.55, p < 0.01. The caloric consumption of savory snack foods was less in the women (107 ± 20 kcal) than the men (275 ± 33 kcal), irrespective of dietary protein level. Conversely, there were no significant main effects, nor an interaction between, gender and dietary protein level for the amount of sweet snack foods consumed. RRVsugar predicted the amount of savory snack foods (F(1,18) = 30.14, p < 0.01), but not the amount of sweet snack foods (F(1,18) = 1.47, p = 0.24), consumed by each participant.
3.4. Habitual Dietary Intake
Habitual dietary intake did not differ prior to each study visit. Average energy intake for the women was 1993 ± 103 kcal/day with a macronutrient composition of 48 ± 2% E carbohydrates, 16 ± 1% E protein, and 35 ± 1% E fat. Habitual protein consumption was 77 ± 5 g protein/day or approximately 1.3 ± 0.1 g protein/kg/day. Daily energy intake for the men was 2240 ± 128 kcal/day with a macronutrient composition of 41 ± 1% E carbohydrates, 21 ± 1% E protein, and 37 ± 2% E fat. Habitual protein consumption was 113 ± 8 g protein/day or approximately 1.5 ± 0.1 g protein/kg/day.
4. Discussion
The present study was conducted to examine the effect of normal (15% E) and high-protein (30% E) meals on the reinforcing values of energy-dense, highly palatable sweet and savory snack foods in healthy weight adults. To our knowledge, this is the first study to test the effect of protein intake on the amount of work an individual is willing to perform to gain access to a highly reinforcing food. Our results show that increasing dietary protein differentially altered food reinforcement in women and men. Most notably, increasing dietary protein intake decreased the reinforcing value of savory foods in women.
The present study is the first to demonstrate an effect of protein intake on the motivation to gain access to a highly rewarding food, as assessed by operant (behavioral) responding, and eating behavior. By demonstrating an effect of dietary protein on behavioral responses, it extends recent neuroimaging studies that have shown that increasing protein intake decreases activation in central motivation and reward areas of the brain; potentially decreasing reward-driven eating [
8,
9,
10]. In overweight/obese late-adolescent girls, a high protein breakfast reduced brain activation responses to visual food cues in the anterior insular and mid-prefrontal cortex 3 h postprandial (pre-lunch) [
8], and in the hippocampus and parahippocampus regions 8 h after breakfast (pre-supper) [
9] compared to a normal protein breakfast (40% E vs. 15% E, respectively). The reduced activation of the aforementioned brain regions coincided with a decrease in nighttime snacking on high-fat foods [
9]. Furthermore, feeding healthy weight women a low-protein (7% E) or high-protein (25% E) diet for 16 days resulted in contrasting postprandial changes in reward-related areas of the brain [
10]. Specifically, increasing dietary protein resulted in decreased activation in the inferior orbitofrontal cortex in response to savory food cues [
10]. Taken together, the results from these neuroimaging studies indicate that dietary protein may modulate reward-driven eating behavior in females. The findings of the current study expand upon the knowledge gained from these neuroimaging studies by demonstrating a significant interaction between dietary protein and gender on motivated behavior for energy-dense savory snack foods. Thus, in women, increasing protein intake modifies eating behavior, specifically, the consumption of savory foods.
The current results indicate that increasing dietary protein from 15% E to 30% E does not alter motivated behavior in men. These findings are supported by those of Frank et al. [
11] who found that changes in neural responses in brain regions responsible for reward-seeking behavior were only visible in women, but not in the men [
11]. Furthermore, Sayer and colleagues [
12] reported no differences in postprandial neural responses to visual food cues 3 h after consuming normal (12 g) and high-protein (25 g) breakfasts in overweight adults (6 women and 12 men). The disproportionate number of women and men in their participant pool may have led to their results more closely resembling the responses that the current and previous study [
11] detailed for men. Taken together, these findings suggest that gender plays an important role in the neurobehavioral responses to dietary protein. Further investigation is necessary to continue to elucidate the effects of gender on eating behavior in response to alterations in dietary macronutrient composition.
Interestingly, increasing dietary protein from 15% E to 30% E did not alter the reinforcing value of sweet foods. This unexpected finding opposes our hypothesis that the reinforcing value of, and consumption of, sweet foods would be lessened after consuming meals containing 30% E compared to 15% E protein. Previous research had shown that increasing dietary protein from 15% E to 40% E [
9] or from 10% E to 25% E [
13] decreases snacking on energy-dense sweet foods. On the other hand, Gosby et al. [
13] reported no measurable differences in the intake of sweet foods when dietary protein was increased from 15% E to 25% E. Therefore, it can be postulated that an increase of at least 15% E, or an increase from a relatively low baseline protein intake of 10% E, is needed to elicit a reduction in the consumption of sweet foods. However, we found no effect of a 15% E increase in dietary protein on the intake of sweet foods. Therefore, a larger divergence between habitual and meal protein intake might be needed in order to alter motivated behavior for sweet foods. Future studies should systematically explore the possibility of a threshold at which motivated behavior is increased or decreased in response to changes in protein intake.
This study is not without limitations. First, only healthy weight adults were recruited for participation. Weight status influences the RRV of food and energy intake [
4], therefore, it is possible that overweight and obese individuals may exhibit different appetitive or motivational responses in response to changes in dietary protein. However, the current study of healthy weight adults provides an initial indication of how dietary protein can alter motivation for, and consumption of, energy-dense sweet and savory snack foods later in the day. Second, only a limited number of snack foods were studied. It is possible that the RRV of snack foods with different taste profiles than those tested here would have produced different results. Still, it is intriguing that increasing dietary protein decreased the RRV of foods with a savory taste profile in the women only.