Familial Resemblance in Dietary Intakes of Children, Adolescents, and Parents: Does Dietary Quality Play a Role?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Interview on Kinship and Household
2.3. Dietary Intake Assessment
2.4. Inclusion Criteria for the Final Study Sample
2.5. Statistical Analysis
3. Results
3.1. Distribution of Family Types
3.2. Characteristics of the Family Members
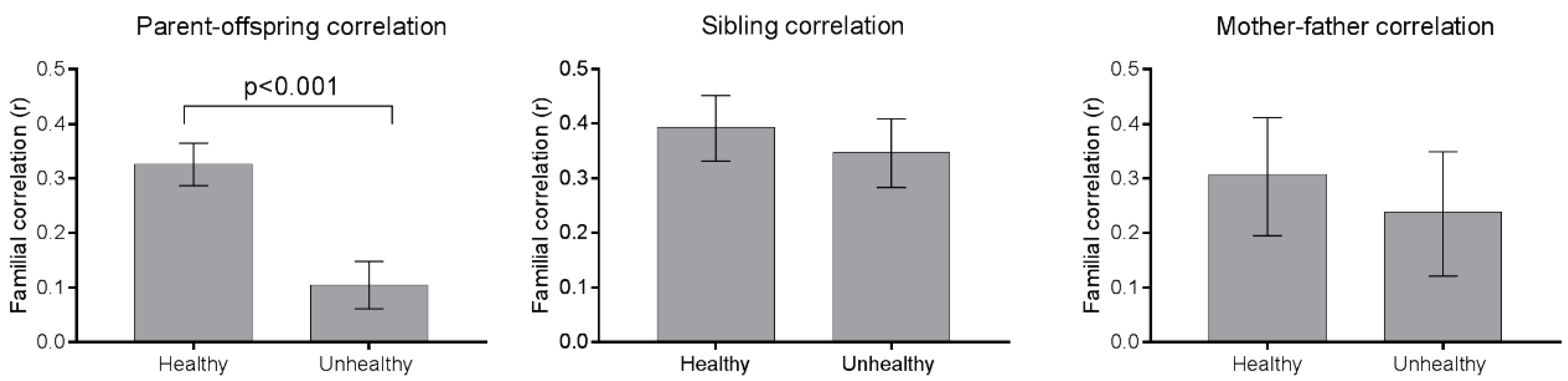
3.3. Familial Correlations
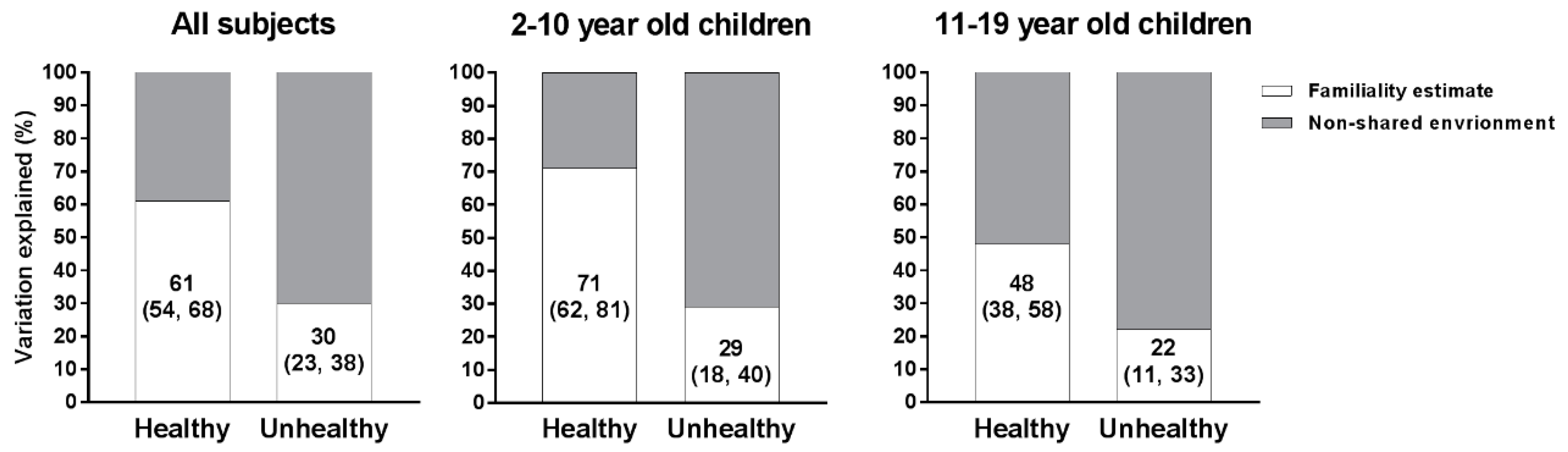
3.4. Familiality Estimates
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- Birch, L.L.; Fisher, J.O. Development of eating behaviors among children and adolescents. Pediatrics 1998, 101, 539–549. [Google Scholar] [PubMed]
- Cullen, K.W.; Baranowski, T.; Owens, E.; Marsh, T.; Rittenberry, L.; de Moor, C. Availability, accessibility, and preferences for fruit, 100% fruit juice, and vegetables influence children’s dietary behavior. Health Educ. Behav. 2003, 30, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Hanson, N.I.; Neumark-Sztainer, D.; Eisenberg, M.E.; Story, M.; Wall, M. Associations between parental report of the home food environment and adolescent intakes of fruits, vegetables and dairy foods. Public Health Nutr. 2005, 8, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.L. Development of food preferences. Annu. Rev. Nutr. 1999, 19, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.L.; McPhee, L.; Shoba, B.C.; Pirok, E.; Steinberg, L. What kind of exposure reduces children’s food neophobia? Looking vs. tasting. Appetite 1987, 9, 171–178. [Google Scholar] [CrossRef]
- Addessi, E.; Galloway, A.T.; Visalberghi, E.; Birch, L.L. Specific social influences on the acceptance of novel foods in 2–5-year-old children. Appetite 2005, 45, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Rifas-Shiman, S.L.; Frazier, A.L.; Rockett, H.R.; Camargo, C.A., Jr.; Field, A.E.; Berkey, C.S.; Colditz, G.A. Family dinner and diet quality among older children and adolescents. Arch. Fam. Med. 2000, 9, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.I.; Neumark-Sztainer, D.; Hannan, P.J.; Story, M. Family meals during adolescence are associated with higher diet quality and healthful meal patterns during young adulthood. J. Am. Diet. Assoc. 2007, 107, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.J.; Larsen, J.K.; Kremers, S.P.; Dagnelie, P.C.; Geenen, R. Peer influence on snacking behavior in adolescence. Appetite 2010, 55, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lytle, L.A.; Seifert, S.; Greenstein, J.; McGovern, P. How do children’s eating patterns and food choices change over time? Results from a cohort study. Am. J. Health Promot. 2000, 14, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.L.; Lee, Y.; Mitchell, D.C.; Smiciklas-Wright, H.; Birch, L.L. The quality of girls’ diets declines and tracks across middle childhood. Int. J. Behav. Nutr. Phys. Act. 2004, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahmann, P.H.; Williams, G.M.; Najman, J.M.; Mamun, A.A. Mother-adult offspring resemblance in dietary intake: A community-based cohort study in Australia. Am. J. Clin. Nutr. 2017, 105, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beydoun, M.A.; Li, J.; Liu, Y.; Moreno, L.A. Do children and their parents eat a similar diet? Resemblance in child and parental dietary intake: Systematic review and meta-analysis. J. Epidemiol. Community Health 2011, 65, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Wang, Y. Parent-child dietary intake resemblance in the United States: Evidence from a large representative survey. Soc. Sci. Med. 2009, 68, 2137–2444. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, W.; Siani, A.; Adan, R.; De Henauw, S.; Eiben, G.; Gwozdz, W.; Hebestreit, A.; Hunsberger, M.; Kaprio, J.; Krogh, V.; et al. Cohort Profile: The transition from childhood to adolescence in European children-how I.Family extends the IDEFICS cohort. Int. J. Epidemiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, W.; Bammann, K.; Siani, A.; Buchecker, K.; De Henauw, S.; Iacoviello, L.; Hebestreit, A.; Krogh, V.; Lissner, L.; Marild, S.; et al. The IDEFICS cohort: Design, characteristics and participation in the baseline survey. Int. J. Obes. 2011, 35 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Hebestreit, A.; Bornhorst, C.; Pala, V.; Barba, G.; Eiben, G.; Veidebaum, T.; Hadjigergiou, C.; Molnar, D.; Claessens, M.; Fernandez-Alvira, J.M.; et al. Dietary energy density in young children across Europe. Int. J. Obes. 2014, 38 (Suppl. S2), S124–S134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vereecken, C.A.; Covents, M.; Sichert-Hellert, W.; Alvira, J.M.; Le Donne, C.; De Henauw, S.; De Vriendt, T.; Phillipp, M.K.; Beghin, L.; Manios, Y.; et al. Development and evaluation of a self-administered computerized 24-h dietary recall method for adolescents in Europe. Int. J. Obes. 2008, 32 (Suppl. S5), S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, C.; Huybrechts, I.; Ahrens, W.; Eiben, G.; Michels, N.; Pala, V.; Molnar, D.; Russo, P.; Barba, G.; Bel-Serrat, S.; et al. Prevalence and determinants of misreporting among European children in proxy-reported 24 h dietary recalls. Br. J. Nutr. 2013, 109, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Midthune, D.; Dodd, K.W.; Freedman, L.S.; Krebs-Smith, S.M.; Subar, A.F.; Guenther, P.M.; Carroll, R.J.; Kipnis, V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J. Am. Diet. Assoc. 2006, 106, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, V.; Midthune, D.; Buckman, D.W.; Dodd, K.W.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Tooze, J.A.; Carroll, R.J.; Freedman, L.S. Modeling data with excess zeros and measurement error: Application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics 2009, 65, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Hebestreit, A.; Intemann, T.; Siani, A.; De Henauw, S.; Eiben, G.; Kourides, Y.A.; Kovacs, E.; Moreno, L.A.; Veidebaum, T.; Krogh, V.; et al. Dietary Patterns of European Children and Their Parents in Association with Family Food Environment: Results from the I.Family Study. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Elston, R.C.; Gray-McGuire, C. A review of the ‘Statistical Analysis for Genetic Epidemiology’ (S.A.G.E.) software package. Hum. Genom. 2004, 1, 456–459. [Google Scholar] [CrossRef]
- Keen, K.J.; Elston, R.C. Robust asymptotic sampling theory for correlations in pedigrees. Stat. Med. 2003, 22, 3229–3247. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Diedenhofen, B.; Musch, J. Cocor: A Comprehensive Solution for the Statistical Comparison of Correlations. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Cocor Comparing Correlations. Available online: http://comparingcorrelations.org/ (accessed on 10 August 2017).
- Almasy, L.; Blangero, J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998, 62, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Neale, M.C. “Familiality” or heritability. Arch. Gen. Psychiatry 2009, 66, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.; Song, Y.; Elston, R. Interval estimation of familial correlations from pedigrees. Stat. Appl. Genet. Mol. Biol. 2011, 10. [Google Scholar] [CrossRef]
- Desor, J.A.; Greene, L.S.; Maller, O. Preferences for sweet and salty in 9- to 15-year-old and adult humans. Science 1975, 190, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Desor, J.A.; Beauchamp, G.K. Longitudinal changes in sweet preferences in humans. Physiol. Behav. 1987, 39, 639–641. [Google Scholar] [CrossRef]
- Lissner, L.; Lanfer, A.; Gwozdz, W.; Olafsdottir, S.; Eiben, G.; Moreno, L.A.; Santaliestra-Pasias, A.M.; Kovacs, E.; Barba, G.; Loit, H.M.; et al. Television habits in relation to overweight, diet and taste preferences in European children: The IDEFICS study. Eur. J. Epidemiol. 2012, 27, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, R.A.; Cassady, D.; Culp, J.; Alcalay, R. Frequency and types of foods advertised on Saturday morning and weekday afternoon English- and Spanish-language American television programs. J. Nutr. Educ. Behav. 2009, 41, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Reisch, L.A.; Gwozdz, W.; Molnar, D.; Konstabel, K.; Michels, N.; Tornaritis, M.; Eiben, G.; Siani, A.; Fernandez-Alvira, J.M.; et al. Pester power and its consequences: Do European children’s food purchasing requests relate to diet and weight outcomes? Public Health Nutr. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Conner, M.; Prestwich, A.; Cavazza, N. The transmission of attitudes towards food: Twofold specificity of similarities with parents and friends. Br. J. Health Psychol. 2012, 17, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Cavazza, N.; Graziani, A.R. Healthy at Home, Unhealthy Outside: Food Groups Associated with Family and Friends and the Potential Impact on Attitude and Consumption. J. Soc. Clin. Psychol. 2014, 33, 343–364. [Google Scholar] [CrossRef]
- Oliveria, S.A.; Ellison, R.C.; Moore, L.L.; Gillman, M.W.; Garrahie, E.J.; Singer, M.R. Parent-child relationships in nutrient intake: The Framingham Children’s Study. Am. J. Clin. Nutr. 1992, 56, 593–598. [Google Scholar] [PubMed]
- Vagstrand, K. Sex differences among Swedish adolescents in mother-child relationships in the intake of different food groups. Br. J. Nutr. 2010, 103, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.I.; Stafleu, A.; de Graaf, C.; van Staveren, W.A. Family resemblance in fat intake in The Netherlands. Eur. J. Clin. Nutr. 1997, 51, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, J.M.; Lluch, A.; Lecomte, E.; Artur, Y.; Herbeth, B. Family resemblance in energy and macronutrient intakes: The Stanislas Family Study. Int. J. Epidemiol. 1996, 25, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.H.; Smeeding, T.M.; Furstenberg, F.F. Are Parents Investing Less Time in Children? Trends in Selected Industrialized Countries. Popul. Dev. Rev. 2004, 30, 647–672. [Google Scholar] [CrossRef]
- Flagg, L.A.; Sen, B.; Kilgore, M.; Locher, J.L. The influence of gender, age, education and household size on meal preparation and food shopping responsibilities. Public Health Nutr. 2014, 17, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Harnack, L.; Story, M.; Martinson, B.; Neumark-Sztainer, D.; Stang, J. Guess who’s cooking? The role of men in meal planning, shopping, and preparation in US families. J. Am. Diet. Assoc. 1998, 98, 995–1000. [Google Scholar] [CrossRef]
- Devine, C.M.; Farrell, T.J.; Blake, C.E.; Jastran, M.; Wethington, E.; Bisogni, C.A. Work conditions and the food choice coping strategies of employed parents. J. Nutr. Educ. Behav. 2009, 41, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.J.; Ness, A.R.; Rogers, I.; Emmett, P.; Cribb, V.; Davey Smith, G.; Lawlor, D.A. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 years: Exploring parental comparisons and prenatal effects. Am. J. Clin. Nutr. 2010, 91, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Musch, J.; Enczmann, J.; Fischer, J. Estimating the prevalence of nonpaternity in Germany. Hum. Nat. 2012, 23, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Voracek, M.; Haubner, T.; Fisher, M.L. Recent decline in nonpaternity rates: A cross-temporal meta-analysis. Psychol. Rep. 2008, 103, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Sellers, T.A.; Kushi, L.H.; Folsom, A.R. Familial correlation of dietary intakes among postmenopausal women. Genet. Epidemiol. 1998, 15, 553–563. [Google Scholar] [CrossRef]
- Silventoinen, K.; Kaprio, J.; Lahelma, E.; Viken, R.J.; Rose, R.J. Assortative mating by body height and BMI: Finnish twins and their spouses. Am. J. Hum. Biol. 2003, 15, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Djafarian, K.; Stewart, J.; Jackson, D.M. Assortative mating for obesity. Am. J. Clin. Nutr. 2007, 86, 316–323. [Google Scholar] [PubMed]
- Agrawal, A.; Heath, A.C.; Grant, J.D.; Pergadia, M.L.; Statham, D.J.; Bucholz, K.K.; Martin, N.G.; Madden, P.A. Assortative mating for cigarette smoking and for alcohol consumption in female Australian twins and their spouses. Behav. Genet. 2006, 36, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.D.; Heath, A.C.; Bucholz, K.K.; Madden, P.A.; Agrawal, A.; Statham, D.J.; Martin, N.G. Spousal concordance for alcohol dependence: Evidence for assortative mating or spousal interaction effects? Alcohol. Clin. Exp. Res. 2007, 31, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Fildes, A.; van Jaarsveld, C.H.; Llewellyn, C.H.; Fisher, A.; Cooke, L.; Wardle, J. Nature and nurture in children’s food preferences. Am. J. Clin. Nutr. 2014, 99, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Fildes, A.; Cooke, L.; Herle, M.; Shakeshaft, N.; Plomin, R.; Llewellyn, C. Genetic and environmental influences on food preferences in adolescence. Am. J. Clin. Nutr. 2016, 104, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Lytle, L.A.; Nichaman, M.Z.; Obarzanek, E.; Glovsky, E.; Montgomery, D.; Nicklas, T.; Zive, M.; Feldman, H. Validation of 24-h recalls assisted by food records in third-grade children. The CATCH Collaborative Group. J. Am. Diet. Assoc. 1993, 93, 1431–1436. [Google Scholar] [CrossRef]
- Sobo, E.J.; Rock, C.L.; Neuhouser, M.L.; Maciel, T.L.; Neumark-Sztainer, D. Caretaker-child interaction during children’s 24-hour dietary recalls: Who contributes what to the recall record? J. Am. Diet. Assoc. 2000, 100, 428–433. [Google Scholar] [CrossRef]
- Hunsberger, M.; Pena, P.; Lissner, L.; Grafstrom, L.; Vanaelst, B.; Bornhorst, C.; Pala, V.; Eiben, G. Validity of self-reported lunch recalls in Swedish school children aged 6–8 years. Nutr. J. 2013, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Family Types | Number of Families | Percentage | Number of Individuals |
|---|---|---|---|
| Mother, 1 child | 536 | 37.4 | 1072 |
| Mother, 2 children | 195 | 13.6 | 585 |
| Mother, 3 children | 10 | 0.7 | 40 |
| Mother, 4 children | 2 | 0.1 | 10 |
| Father, 1 child | 129 | 9.0 | 258 |
| Father, 2 children | 38 | 2.7 | 114 |
| Father, 3 children | 6 | 0.4 | 24 |
| Father, 4 children | 1 | 0.1 | 5 |
| Mother, father, 1 child | 154 | 10.7 | 462 |
| Mother, father, 2 children | 89 | 6.2 | 356 |
| Mother, father, 3 children | 18 | 1.3 | 90 |
| Mother, father, 4 children | 3 | 0.2 | 18 |
| 2 children | 236 | 16.45 | 472 |
| 3 children | 18 | 1.15 | 54 |
| Total | 1435 | 100 | 3560 |
| Mothers | Fathers | Daughters 2–10 Year-Old | Sons 2–10 Year-Old | Daughters 11–19 Year-Old | Sons 11–19 Year-Old | |
|---|---|---|---|---|---|---|
| N, number of subjects | 1007 | 438 | 517 | 551 | 518 | 529 |
| Total energy (kcal) | 1707 ± 154 | 2254 ± 192 | 1605 ± 131 | 1760 ± 182 | 1809 ± 141 | 2082 ± 204 |
| Macronutrients (% energy intake) | ||||||
| Total fat | 37 ± 4 | 36 ± 4 | 33 ± 3 | 33 ± 3 | 34 ± 3 | 33 ± 3 |
| Total carbohydrates | 45 ± 5 | 44 ± 5 | 52 ± 4 | 52 ± 4 | 51 ± 4 | 51 ± 4 |
| Total protein | 16 ± 2 | 16 ± 1 | 15 ± 1 | 15 ± 1 | 15 ± 1 | 15 ± 1 |
| Total sugar | 19 ± 5 | 17 ± 4 | 21 ± 5 | 21 ± 4 | 20 ± 4 | 20 ± 3 |
| Healthy food groups (g/1000 kcal) | ||||||
| Healthy cereals and cereal products | 35 ± 20 | 35 ± 22 | 22 ± 14 | 25 ± 13 | 21 ± 14 | 20 ± 10 |
| Healthy fat and oils | 10 ± 4 | 10 ± 4 | 11 ± 4 | 10 ± 3 | 10 ± 3 | 10 ± 3 |
| Fruits and vegetables | 155 ± 63 | 113 ± 47 | 97 ± 35 | 88 ± 31 | 89 ± 31 | 70 ± 27 |
| Healthy meat and meat products | 27 ± 5 | 26 ± 6 | 24 ± 5 | 23 ± 5 | 22 ± 5 | 24 ± 6 |
| Healthy milk and dairy products | 57 ± 42 | 50 ± 36 | 74 ± 60 | 81 ± 59 | 65 ± 52 | 64 ± 48 |
| Healthy meat alternatives | 23 ± 13 | 22 ± 11 | 15 ± 7 | 16 ± 10 | 12 ± 6 | 14 ± 10 |
| Healthy mixed dishes, | 57 ± 14 | 55 ± 8 | 46 ± 9 | 43 ± 11 | 51 ± 8 | 47 ± 10 |
| Unhealthy food groups (g/1000 kcal) | ||||||
| Unhealthy cereals and cereal products | 48 ± 14 | 55 ± 21 | 77 ± 18 | 76 ± 20 | 70 ± 16 | 78 ± 19 |
| Unhealthy fat and oils | 11 ± 5 | 10 ± 5 | 9 ± 3 | 11 ± 4 | 10 ± 3 | 10 ± 3 |
| Sugar and sweets | 46 ± 22 | 37 ± 19 | 49 ± 22 | 47 ± 12 | 47 ± 22 | 41 ± 11 |
| Unhealthy meat and meat products | 23 ± 5 | 31 ± 4 | 29 ± 5 | 28 ± 4 | 27 ± 5 | 27 ± 4 |
| Unhealthy milk and dairy products | 56 ± 42 | 46 ± 39 | 68 ± 41 | 70 ± 50 | 56 ± 32 | 57 ± 43 |
| Unhealthy mixed dishes | 56 ± 8 | 53 ± 8 | 53 ± 6 | 54 ± 7 | 60 ± 6 | 60 ± 7 |
| Sum of healthy and unhealthy food groups (g/1000 kcal) | ||||||
| Healthy foods | 364 ± 89 | 311 ± 67 | 289 ± 80 | 285 ± 76 | 271 ± 69 | 249 ± 64 |
| Unhealthy foods | 240 ± 47 | 231 ± 45 | 285 ± 46 | 285 ± 50 | 271 ± 39 | 273 ± 45 |
| Parent–Offspring (n = 1987) | Sibling-Sibling (n = 750) | Parent–Offspring 2–10 Year-Old (n = 996) | Parent–Offspring 11–19 Year-Old (n = 991) | Sibling-Sibling 2–10 Year-Old (n = 192) | Sibling-Sibling 11–19 Year-Old (n = 149) | Mother-Father (n = 264) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | r | p-Value a | r | r | p-Value a | r | r | p-Value a | r | |
| Total energy (kcal) | 0.16 | 0.28 | 0.004 | 0.13 | 0.17 | 0.38 | 0.38 | 0.14 | 0.02 | 0.23 |
| Macronutrients (% energy intake) | ||||||||||
| Total fat | 0.21 | 0.32 | 0.01 | 0.21 | 0.22 | 0.92 | 0.31 | 0.26 | 0.63 | 0.25 |
| Total carbohydrates | 0.21 | 0.31 | 0.02 | 0.18 | 0.24 | 0.19 | 0.33 | 0.32 | 0.90 | 0.26 |
| Total protein | 0.26 | 0.31 | 0.23 | 0.25 | 0.26 | 0.81 | 0.31 | 0.22 | 0.37 | 0.33 |
| Total sugar | 0.21 | 0.34 | <0.001 | 0.22 | 0.18 | 0.38 | 0.37 | 0.17 | 0.05 | 0.26 |
| Healthy food groups (gram/1000 kcal) | ||||||||||
| Healthy cereals and cereal products | 0.33 | 0.38 | 0.18 | 0.36 | 0.29 | 0.11 | 0.48 | 0.36 | 0.18 | 0.27 |
| Healthy fat and oils | 0.27 | 0.34 | 0.09 | 0.31 | 0.24 | 0.06 | 0.45 | 0.31 | 0.14 | 0.23 |
| Fruits and vegetables | 0.33 | 0.35 | 0.59 | 0.38 | 0.28 | 0.01 | 0.43 | 0.19 | 0.02 | 0.29 |
| Healthy meat and meat products | 0.21 | 0.40 | <0.001 | 0.23 | 0.18 | 0.20 | 0.31 | 0.24 | 0.49 | 0.27 |
| Healthy milk and dairy products | 0.25 | 0.36 | 0.004 | 0.28 | 0.23 | 0.26 | 0.52 | 0.35 | 0.06 | 0.29 |
| Healthy meat alternatives | 0.23 | 0.21 | 0.67 | 0.26 | 0.21 | 0.19 | 0.39 | 0.30 | 0.41 | 0.29 |
| Healthy mixed dishes | 0.23 | 0.43 | <0.001 | 0.25 | 0.19 | 0.18 | 0.52 | 0.30 | 0.02 | 0.22 |
| Unhealthy food groups (gram/1000 kcal) | ||||||||||
| Unhealthy cereals and cereal products | 0.23 | 0.33 | 0.02 | 0.22 | 0.22 | 0.86 | 0.45 | 0.28 | 0.07 | 0.27 |
| Unhealthy fat and oils | 0.22 | 0.39 | <0.001 | 0.25 | 0.19 | 0.24 | 0.50 | 0.27 | 0.02 | 0.17 |
| Sugar and sweets | 0.19 | 0.31 | 0.003 | 0.22 | 0.19 | 0.37 | 0.40 | 0.26 | 0.17 | 0.24 |
| Unhealthy meat and meat products | 0.20 | 0.37 | <0.001 | 0.20 | 0.21 | 0.77 | 0.43 | 0.35 | 0.43 | 0.17 |
| Unhealthy milk and dairy products | 0.11 | 0.39 | <0.001 | 0.12 | 0.10 | 0.63 | 0.42 | 0.30 | 0.25 | 0.21 |
| Unhealthy mixed dishes | 0.20 | 0.34 | <0.001 | 0.19 | 0.19 | 0.84 | 0.46 | 0.20 | 0.01 | 0.15 |
| Parent–Offspring | Siblings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Father–Son (n = 326) | Mother–Son (n = 705) | Father–Daughter (n = 299) | Mother–Daughter (n = 657) | Brother–Brother (n = 194) | Sister–Brother (n = 358) | Sister–Sister (n = 198) | |||
| r | r | R | r | p-Value a | r | r | r | p-Value a | |
| Total energy (kcal) | 0.18 | 0.14 | 0.16 | 0.17 | 0.95 | 0.40 | 0.20 | 0.30 | 0.05 |
| Macronutrients (% energy intake) | |||||||||
| Total fat | 0.16 | 0.20 | 0.27 | 0.25 | 0.39 | 0.33 | 0.31 | 0.32 | 0.96 |
| Total carbohydrates | 0.14 | 0.22 | 0.20 | 0.25 | 0.41 | 0.31 | 0.33 | 0.32 | 0.98 |
| Total protein | 0.23 | 0.26 | 0.28 | 0.25 | 0.93 | 0.38 | 0.24 | 0.32 | 0.26 |
| Total sugar | 0.09 | 0.22 | 0.25 | 0.22 | 0.43 | 0.39 | 0.29 | 0.35 | 0.42 |
| Healthy food groups (gram/1000 kcal) | |||||||||
| Healthy cereals and cereal products | 0.28 | 0.35 | 0.30 | 0.33 | 0.66 | 0.43 | 0.33 | 0.41 | 0.38 |
| Healthy fat and oils | 0.27 | 0.30 | 0.14 | 0.31 | 0.10 | 0.33 | 0.34 | 0.31 | 0.91 |
| Fruits and vegetables | 0.31 | 0.30 | 0.38 | 0.35 | 0.57 | 0.35 | 0.32 | 0.40 | 0.57 |
| Healthy meat and meat products | 0.32 | 0.24 | 0.17 | 0.21 | 0.25 | 0.30 | 0.20 | 0.11 b | 0.18 |
| Healthy milk and dairy products | 0.21 | 0.23 | 0.18 | 0.20 | 0.88 | 0.42 | 0.37 | 0.38 | 0.40 |
| Healthy meat alternatives | 0.21 | 0.32 | 0.19 | 0.22 | 0.004 | 0.53 | 0.37 | 0.22 | 0.002 |
| Healthy mixed dishes | 0.15 | 0.23 | 0.13 | 0.26 | 0.08 | 0.51 | 0.26 | 0.49 | 0.005 |
| Unhealthy food groups (gram/1000 kcal) | |||||||||
| Unhealthy cereals and cereal products | 0.28 | 0.24 | 0.25 | 0.23 | 0.99 | 0.31 | 0.32 | 0.38 | 0.38 |
| Unhealthy fat and oils | 0.21 | 0.27 | 0.15 | 0.21 | 0.37 | 0.49 | 0.34 | 0.38 | 0.11 |
| Sugar and sweets | 0.21 | 0.21 | 0.16 | 0.18 | 0.87 | 0.36 | 0.22 | 0.39 | 0.16 |
| Unhealthy meat and meat products | 0.06 b | 0.24 | 0.03 b | 0.25 | <0.001 | 0.46 | 0.37 | 0.33 | 0.39 |
| Unhealthy milk and dairy products | 0.06 b | 0.11 | 0.06 b | 0.15 | 0.51 | 0.37 | 0.33 | 0.38 | 0.92 |
| Unhealthy mixed dishes | 0.15 | 0.16 | 0.13 | 0.30 | 0.02 | 0.44 | 0.30 | 0.31 | 0.17 |
| All Subjects (n = 3560) | Children 2–10 Year-Old (n = 1777) | Children 11–19 Year-Old (n = 1773) | |
|---|---|---|---|
| Familiality (95% CI) a | Familiality (95% CI) | Familiality (95% CI) | |
| Total energy (kcal) | 0.35 (0.27, 0.42) | 0.32 (0.22, 0.43) | 0.29 (0.18, 0.40) |
| Macronutrients (% energy intake) | |||
| Total fat | 0.46 (0.39, 0.53) | 0.43 (0.32, 0.54) | 0.43 (0.32, 0.54) |
| Total carbohydrates | 0.44 (0.37, 0.51) | 0.39 (0.28, 0.50) | 0.44 (0.33, 0.54) |
| Total protein | 0.47 (0.40, 0.54) | 0.48 (0.38, 0.58) | 0.44 (0.34, 0.55) |
| Total sugar | 0.44 (0.36, 0.51) | 0.44 (0.34, 0.55) | 0.33 (0.22, 0.45) |
| Healthy food groups (gram/1000 kcal) | |||
| Healthy cereals and cereal products | 0.59 (0.53, 0.66) | 0.64 (0.55, 0.73) | 0.53 (0.43, 0.63) |
| Healthy fat and oils | 0.52 (0.45, 0.59) | 0.61 (0.51, 0.71) | 0.43 (0.32, 0.53) |
| Fruits and vegetables | 0.61 (0.54, 0.68) | 0.72 (0.62, 0.81) | 0.50 (0.39, 0.60) |
| Healthy meat and meat products | 0.41 (0.33, 0.48) | 0.46 (0.36, 0.57) | 0.38 (0.27, 0.49) |
| Healthy milk and dairy products | 0.45 (0.38, 0.52) | 0.49 (0.39, 0.60) | 0.34 (0.24, 0.44) |
| Healthy meat alternatives | 0.42 (0.35, 0.49) | 0.52 (0.42, 0.62) | 0.43 (0.32, 0.54) |
| Healthy mixed dishes | 0.48 (0.41, 0.55) | 0.49 (0.39, 0.59) | 0.38 (0.27, 0.50) |
| Unhealthy food groups (gram/1000 kcal) | |||
| Unhealthy cereals and cereal products | 0.46 (0.38, 0.53) | 0.43 (0.33, 0.53) | 0.41 (0.30, 0.52) |
| Unhealthy fat and oils | 0.46 (0.39, 0.53) | 0.50 (0.40, 0.60) | 0.37 (0.27, 0.48) |
| Sugar and sweets | 0.42 (0.34, 0.49) | 0.50 (0.39, 0.60) | 0.34 (0.23, 0.45) |
| Unhealthy meat and meat products | 0.44 (0.37, 0.51) | 0.41 (0.31, 0.52) | 0.41 (0.30, 0.52) |
| Unhealthy milk and dairy products | 0.31 (0.24, 0.38) | 0.29 (0.18, 0.40) | 0.21 (0.10, 0.32) |
| Unhealthy mixed dishes | 0.42 (0.35, 0.49) | 0.39 (0.29, 0.49) | 0.38 (0.27, 0.50) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogl, L.H.; Silventoinen, K.; Hebestreit, A.; Intemann, T.; Williams, G.; Michels, N.; Molnár, D.; Page, A.S.; Pala, V.; Papoutsou, S.; et al. Familial Resemblance in Dietary Intakes of Children, Adolescents, and Parents: Does Dietary Quality Play a Role? Nutrients 2017, 9, 892. https://doi.org/10.3390/nu9080892
Bogl LH, Silventoinen K, Hebestreit A, Intemann T, Williams G, Michels N, Molnár D, Page AS, Pala V, Papoutsou S, et al. Familial Resemblance in Dietary Intakes of Children, Adolescents, and Parents: Does Dietary Quality Play a Role? Nutrients. 2017; 9(8):892. https://doi.org/10.3390/nu9080892
Chicago/Turabian StyleBogl, Leonie H., Karri Silventoinen, Antje Hebestreit, Timm Intemann, Garrath Williams, Nathalie Michels, Dénes Molnár, Angie S. Page, Valeria Pala, Stalo Papoutsou, and et al. 2017. "Familial Resemblance in Dietary Intakes of Children, Adolescents, and Parents: Does Dietary Quality Play a Role?" Nutrients 9, no. 8: 892. https://doi.org/10.3390/nu9080892






