Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedures
2.3. Routine Clinical Care
2.4. Definitions
2.5. Statistical Analyses
3. Results
3.1. Baseline Pharmacological and Nutritional Characteristics
3.2. Pharmacological and Nutritional Management in BP-On Target (BP-OT) and BP-Not On Target (BP-NOT) Groups
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- Tancredi, M.; Rosengren, A.; Svensson, A.M.; Kosiborod, M.; Pivodic, A.; Gudbjornsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Coady, S.; Sorlie, P.D.; Levy, D.; Meigs, J.B.; D’Agostino, R.B., Sr.; Wilson, P.W.; Savage, P.J. Trends in Cardiovascular Complications of Diabetes. JAMA 2004, 292, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Freiberg, J.J.; Nordestgaard, B.G. Extent of Undertreatment and Overtreatment with Cholesterol-Lowering Therapy According to European Guidelines in 92,348 Danes without Ischemic Cardiovascular Disease and Diabetes in 2004–2014. Atherosclerosis 2016, 257, 9–15. [Google Scholar] [CrossRef] [PubMed]
- De Cosmo, S.; Viazzi, F.; Piscitelli, P.; Giorda, C.; Ceriello, A.; Genovese, S.; Russo, G.; Guida, P.; Fioretto, P.; Pontremoli, R.; et al. Blood Pressure Status and the Incidence of Diabetic Kidney Disease in Patients with Hypertension and Type 2 Diabetes. J. Hypertens. 2016, 34, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Gorter, K.; van Bruggen, R.; Stolk, R.; Zuithoff, P.; Verhoeven, R.; Rutten, G. Overall Quality of Diabetes Care in a Defined Geographic Region: Different Sides of the Same Story. Br. J. Gen. Pract. 2008, 58, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Baptista, D.R.; Thieme, R.D.; Reis, W.C.; Pontarolo, R.; Correr, C.J. Proportion of Brazilian Diabetes Patients that Achieve Treatment Goals: Implications for Better Quality of Care. Diabetol. Metab. Syndr. 2015, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Laxy, M.; Knoll, G.; Schunk, M.; Meisinger, C.; Huth, C.; Holle, R. Quality of Diabetes Care in Germany Improved from 2000 to 2007 to 2014, but Improvements Diminished since 2007. Evidence from the Population-Based KORA Studies. PLoS ONE 2016, 11, e0164704. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.M. Measuring Population Sodium Intake: A Review of Methods. Nutrients 2014, 6, 4651–4662. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Sodium Intake for Adults and Children; WHO Press: Geneva, Switzerland, 2012. [Google Scholar]
- Fox, C.S.; Golden, S.H.; Anderson, C.; Bray, G.A.; Burke, L.E.; de Boer, I.H.; Deedwania, P.; Eckel, R.H.; Ershow, A.G.; Fradkin, J.; et al. Update on Prevention of Cardiovascular Disease in Adults with Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2015, 38, 1777–1803. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Violi, F.; D’Amico, R.; Vinceti, M. The Effect of Potassium Supplementation on Blood Pressure in Hypertensive Subjects: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 230, 127–135. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Potassium Intake for Adults and Children; WHO Press: Geneva, Switzerland, 2012. [Google Scholar]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Bain, L.K.; Myint, P.K.; Jennings, A.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Welch, A.A. The Relationship between Dietary Magnesium Intake, Stroke and its Major Risk Factors, Blood Pressure and Cholesterol, in the EPIC-Norfolk Cohort. Int. J. Cardiol. 2015, 196, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.M.; Gansevoort, R.T.; Mukamal, K.J.; Kootstra-Ros, J.E.; Feskens, E.J.; Geleijnse, J.M.; Navis, G.; Bakker, S.J. PREVEND Study Group. Urinary Magnesium Excretion and Risk of Hypertension: The Prevention of Renal and Vascular End-Stage Disease Study. Hypertension 2013, 61, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Bozic, M.; Panizo, S.; Sevilla, M.A.; Riera, M.; Soler, M.J.; Pascual, J.; Lopez, I.; Freixenet, M.; Fernandez, E.; Valdivielso, J.M. High Phosphate Diet Increases Arterial Blood Pressure Via a Parathyroid Hormone Mediated Increase of Renin. J. Hypertens. 2014, 32, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Mitchell, J.H.; Crawford, S.; Huang, C.L.; Maalouf, N.; Hu, M.C.; Moe, O.W.; Smith, S.A.; Vongpatanasin, W. High Dietary Phosphate Intake Induces Hypertension and Augments Exercise Pressor Reflex Function in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R39–R48. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A Formula to Estimate the Approximate Surface Area if Height and Weight be Known. 1916. Nutrition 1989, 5, 303–311. [Google Scholar] [PubMed]
- Wendel-Vos, G.C.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and Relative Validity of the Short Questionnaire to Assess Health-Enhancing Physical Activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; Ryden, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; et al. ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD: The Task Force on Diabetes, Pre-Diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and Developed in Collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 337–414. [Google Scholar]
- Kromhout, D.; Spaaij, C.J.; de Goede, J.; Weggemans, R.M. The 2015 Dutch Food-Based Dietary Guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Gezondheidsraad. Richtlijnen Goede Voeding 2015; Gezondheidsraad: The Hague, The Netherlands, 2015. [Google Scholar]
- Bock, H.A.; Stein, J.H. Diuretics and the Control of Extracellular Fluid Volume: Role of Counterregulation. Semin. Nephrol. 1988, 8, 264–272. [Google Scholar] [PubMed]
- Holbrook, J.T.; Patterson, K.Y.; Bodner, J.E.; Douglas, L.W.; Veillon, C.; Kelsay, J.L.; Mertz, W.; Smith, J.C., Jr. Sodium and Potassium Intake and Balance in Adults Consuming Self-Selected Diets. Am. J. Clin. Nutr. 1984, 40, 786–793. [Google Scholar] [PubMed]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A Method for Estimating Nitrogen Intake of Patients with Chronic Renal Failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Newsome, B.; Ix, J.H.; Tighiouart, H.; Sarnak, M.J.; Levey, A.S.; Beck, G.J.; Block, G. Effect of Protein Restriction on Serum and Urine Phosphate in the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2013, 61, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Wheeler, D.C.; Persky, M.S.; Kestenbaum, B.; Ketteler, M.; Spiegel, D.M.; Allison, M.A.; Asplin, J.; Smits, G.; Hoofnagle, A.N.; et al. Effects of Phosphate Binders in Moderate CKD. J. Am. Soc. Nephrol. 2012, 23, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Marso, S.P.; Bain, S.C.; Franek, E.; Jacob, S.; Masmiquel, L.; Leiter, L.A.; Haluzik, M.; Satman, I.; Omar, M.; et al. LEADER-4: Blood Pressure Control in Patients with Type 2 Diabetes and High Cardiovascular Risk: Baseline Data from the LEADER Randomized Trial. J. Hypertens. 2016, 34, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Halimi, J.M.; Joly, D.; Combe, C.; Choukroun, G.; Dussol, B.; Fauvel, J.P.; Quere, S.; Fiquet, B. Blood Pressure and Proteinuria Control Remains a Challenge in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease: Experience from the Prospective Observational ALICE-PROTECT Study. BMC Nephrol. 2016, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.P.; Sidorenkov, G.; Kleefstra, N.; Bouma, M.; Meulepas, M.; Voorham, J.; Navis, G.; Bilo, H.J.; Denig, P. Development and Validation of Prescribing Quality Indicators for Patients with Type 2 Diabetes. Int. J. Clin. Pract. 2017, 71. [Google Scholar] [CrossRef] [PubMed]
- Walraven, I.; Mast, M.R.; Hoekstra, T.; Jansen, A.P.; Rauh, S.P.; Rutters, F.R.; van der Heijden, A.A.; Elders, P.J.; Moll, A.C.; Polak, B.C.; et al. Real-World Evidence of Suboptimal Blood Pressure Control in Patients with Type 2 Diabetes. J. Hypertens. 2015, 33, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, B.; Antoniou, S.; Burnier, M.; de la Sierra, A.; Volpe, M. Current Situation of Medication Adherence in Hypertension. Front. Pharmacol. 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- De Jager, R.L.; de Beus, E.; Beeftink, M.M.; Sanders, M.F.; Vonken, E.J.; Voskuil, M.; van Maarseveen, E.M.; Bots, M.L.; Blankestijn, P.J. SYMPATHY Investigators. Impact of Medication Adherence on the Effect of Renal Denervation: The SYMPATHY Trial. Hypertension 2017, 69, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Dunkler, D.; Kohl, M.; Heinze, G.; Teo, K.K.; Rosengren, A.; Pogue, J.; Gao, P.; Gerstein, H.; Yusuf, S.; Oberbauer, R.; et al. Modifiable Lifestyle and Social Factors Affect Chronic Kidney Disease in High-Risk Individuals with Type 2 Diabetes Mellitus. Kidney Int. 2015, 87, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention Or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected Effect of Dietary Salt Reductions on Future Cardiovascular Disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M.; Arcand, J.; Leung, A.A.; Thout, S.R.; Campbell, N.R.; Webster, J. The Science of Salt: A Regularly Updated Systematic Review of Salt and Health Outcomes (December 2015–March 2016). J. Clin. Hypertens. (Greenwich) 2017, 19, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, M.; Etemad, Z.; van den Bogaard, C.H.; van der A, D.L. Salt, Iodine and Potassium Intake among Adults in Doetinchem in 2015; RIVM Report 2016–0081; Rijksinstituut voor Volksgezondheid en Milieu: Bilthoven, The Netherlands, 2016.
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of Urinary Sodium and Potassium Excretion with Blood Pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kwakernaak, A.J.; Krikken, J.A.; Binnenmars, S.H.; Visser, F.W.; Hemmelder, M.H.; Woittiez, A.J.; Groen, H.; Laverman, G.D.; Navis, G. Holland Nephrology Study (HONEST) Group. Effects of Sodium Restriction and Hydrochlorothiazide on RAAS Blockade Efficacy in Diabetic Nephropathy: A Randomised Clinical Trial. Lancet Diabetes Endocrinol. 2014, 2, 385–395. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; de Borst, M.H.; Bakker, S.J.; Navis, G.J. Improving the Efficacy of RAAS Blockade in Patients with Chronic Kidney Disease. Nat. Rev. Nephrol. 2013, 9, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Waanders, F.; Boomsma, F.; de Zeeuw, D.; Navis, G. Effects of Dietary Sodium and Hydrochlorothiazide on the Antiproteinuric Efficacy of Losartan. J. Am. Soc. Nephrol. 2008, 19, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; Holtkamp, F.A.; Parving, H.H.; Navis, G.J.; Lewis, J.B.; Ritz, E.; de Graeff, P.A.; de Zeeuw, D. Moderation of Dietary Sodium Potentiates the Renal and Cardiovascular Protective Effects of Angiotensin Receptor Blockers. Kidney Int. 2012, 82, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Gansevoort, R.T.; Mukamal, K.J.; de Boer, R.A.; Navis, G.; Bakker, S.J.; Joosten, M.M. Urinary Potassium Excretion and Risk of Developing Hypertension: The Prevention of Renal and Vascular End-Stage Disease Study. Hypertension 2014, 64, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, O.V.; Yogeswaran, P.; Longo-Mbenza, B.; Ter Goon, D. Uncontrolled Hypertension and its Determinants in Patients with Concomitant Type 2 Diabetes Mellitus (T2DM) in Rural South Africa. PLoS ONE 2016, 11, e0150033. [Google Scholar] [CrossRef] [PubMed]
- Zomer, E.; Gurusamy, K.; Leach, R.; Trimmer, C.; Lobstein, T.; Morris, S.; James, W.P.; Finer, N. Interventions that Cause Weight Loss and the Impact on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Obes. Rev. 2016, 17, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Garg, R. Effect of Magnesium Supplementation on Type 2 Diabetes Associated Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2017. [Google Scholar] [CrossRef] [PubMed]
- Humalda, J.K.; Keyzer, C.A.; Binnenmars, S.H.; Kwakernaak, A.J.; Slagman, M.C.; Laverman, G.D.; Bakker, S.J.; de Borst, M.H.; Navis, G.J. Concordance of Dietary Sodium Intake and Concomitant Phosphate Load: Implications for Sodium Interventions. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.M.; Gansevoort, R.T.; Mukamal, K.J.; van der Harst, P.; Geleijnse, J.M.; Feskens, E.J.; Navis, G.; Bakker, S.J.; PREVEND Study Group. Urinary and Plasma Magnesium and Risk of Ischemic Heart Disease. Am. J. Clin. Nutr. 2013, 97, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Grams, M.E. Serum Phosphorus and Mortality in the Third National Health and Nutrition Examination Survey (NHANES III): Effect Modification by Fasting. Am. J. Kidney Dis. 2014, 64, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Palomino, H.L.; Rifkin, D.E.; Anderson, C.; Criqui, M.H.; Whooley, M.A.; Ix, J.H. 24-Hour Urine Phosphorus Excretion and Mortality and Cardiovascular Events. Clin. J. Am. Soc. Nephrol. 2013, 8, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate Additives in Food—A Health Risk. Dtsch. Arzteblatt Int. 2012, 109, 49–55. [Google Scholar]
- Kieneker, L.M.; Bakker, S.J.; de Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low Potassium Excretion but Not High Sodium Excretion is Associated with Increased Risk of Developing Chronic Kidney Disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, Y.; Hoekstra, T.; Dekker, F.W.; Navis, G.; Vogt, L.; van der Boog, P.J.M.; Bos, W.J.W.; van Montfrans, G.A.; van Dijk, S.; ESMO Study Group. Sodium Restriction in Patients with CKD: A Randomized Controlled Trial of Self-Management Support. Am. J. Kidney Dis. 2017, 69, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Van Zuilen, A.D.; Bots, M.L.; Dulger, A.; van der Tweel, I.; van Buren, M.; Ten Dam, M.A.; Kaasjager, K.A.; Ligtenberg, G.; Sijpkens, Y.W.; Sluiter, H.E.; et al. Multifactorial Intervention with Nurse Practitioners does Not Change Cardiovascular Outcomes in Patients with Chronic Kidney Disease. Kidney Int. 2012, 82, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.P.J.; Sidorenkov, G.; Navis, G.; Bouma, M.; Meulepas, M.A.; Bilo, H.J.G.; Denig, P. Prescribing Quality and Prediction of Clinical Outcomes in Patients with Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care 2017, 40, e83–e84. [Google Scholar] [CrossRef] [PubMed]

| eGFR ≥60 | eGFR <60 | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Total Population | Albuminuria No | Albuminuria Yes | Albuminuria No | Albuminuria Yes | p-Value | |
| Number of patients (% of population) | 450 | 257 (57) | 85 (19) | 52 (12) | 51 (11) | ||
| Patient characteristics | |||||||
| Age (years) | 63 ± 9 | 61 ± 9 | 62 ± 8 | 67 ± 8 *,† | 69 ± 7 *,† | <0.001 | |
| Male, n (%) | 259 (58) | 139 (54) | 63 (74) | 19 (37) | 39 (77) | <0.001 | |
| Years T2DM (years) | 11 (7–18) | 11 (7–18) | 14 (8–19) | 12 (6–17) | 10 (6–15) | 0.45 | |
| Serum HbA1C (mmol/mol) | 57 ± 12 | 58 ± 11 | 59 ± 13 | 54 ± 11 | 57 ± 13 | 0.15 | |
| Insulin use, n (%) | 284 (63) | 160 (62) | 64 (75) | 31 (60) | 28 (55) | 0.07 | |
| Systolic blood pressure (mmHg) | 139 ± 16 | 136 ± 15 | 140 ± 19 | 131 ± 13 † | 139 ± 17 | 0.009 | |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 75 ± 9 | 76 ± 10 | 70 ± 9 *,† | 75 ± 10 ‡ | 0.004 | |
| BP on target, n (%) | 236 (53) | 155 (60) | 28 (33) | 41 (79) | 12 (24) | <0.001 | |
| Macrovascular disease, n (%) | 158 (35) | 68 (27) | 36 (42) | 25 (48) | 31 (61) | <0.001 | |
| eGFR (mL/min) | 84 (62–97) | 92 (78–100) | 88 (74–99) | 47 (36–54) | 39 (33–45) | <0.001 | |
| Albumin excretion (mg/day) | 11 (3–66) | 5 (2–11) | 94 (62–202) | 4 (1–12) | 332 (93–661) | <0.001 | |
| Pharmacological management | |||||||
| RAASi, n (%) | 296 (67) | 152 (59) | 63 (74) | 39 (75) | 42 (82) | 0.001 | |
| β-blockers, n (%) | 207 (46) | 100 (39) | 37 (44) | 36 (69) | 33 (65) | <0.001 | |
| Thiazide diuretics, n (%) | 137 (31) | 81 (32) | 15 (18) | 21 (40) | 18 (35) | 0.02 | |
| Calcium antagonists, n (%) | 101 (23) | 43 (17) | 26 (31) | 13 (25) | 19 (37) | 0.002 | |
| Loop diuretics, n (%) | 81 (18) | 26 (10) | 18 (21) | 17 (33) | 20 (39) | <0.001 | |
| Potassium sparing diuretics, n (%) | 43 (10) | 11 (4) | 8 (9) | 12 (23) | 12 (24) | <0.001 | |
| Number of antihypertensives | 2 (1–3) | 2 (0–3) | 2 (1–3) | 3 (2–3) | 3 (2–4) | <0.001 | |
| No antihypertensive therapy, n (%) | 83 (19) | 65 (25) | 12 (14) | 1 (2) | 2 (4) | <0.001 | |
| 1 drug, n (%) | 101 (23) | 61 (24) | 17 (20) | 6 (12) | 6 (12) | ||
| 2 drugs, n (%) | 106 (24) | 57 (22) | 28 (33) | 13 (25) | 11 (22) | ||
| 3 drugs, n (%) | 91 (20) | 44 (17) | 15 (18) | 21 (40) | 12 (24) | ||
| 4 drugs, n (%) | 56 (13) | 24 (9) | 10 (12) | 8 (15) | 13 (26) | ||
| 5+ drugs, n (%) | 11 (3) | 6 (2) | 3 (4) | 3 (6) | 7 (14) | ||
| Hypertension requiring 4+ drugs, n (%) | 117 (26) | 48 (19) | 23 (27) | 16 (31) | 30 (59) | <0.001 | |
| Total number of drugs | 7 ± 3 | 6 ± 3 | 7 ± 2 | 8 ± 3 * | 9 ± 3 *,† | <0.001 | |
| Non-pharmacological management | |||||||
| BMI (kg/m2) | 32.9 ± 6.2 | 32.9 ± 6.5 | 32.9 ± 5.4 | 33.3 ± 6.2 | 32.3 ± 6.1 | 0.89 | |
| Current smoker, n (%) | 74 (17) | 41 (16) | 15 (18) | 10 (19) | 8 (16) | 0.93 | |
| Alcohol intake (units per month) | 5 (0–30) | 5 (0–28) | 10 (0–47) | 3 (0–24) | 12 (0–40) | 0.22 | |
| 25(OH) Vitamin D (nmol/L) | 42 ± 20 | 43 ± 18 | 37 ± 19 | 42 ± 26 | 44 ± 22 | 0.09 | |
| Urinary excretion | |||||||
| Urinary creatinine excretion (mmol/day) | 13.8 ± 4.8 | 13.9 ± 4.9 | 14.8 ± 5.4 | 12.8 ± 4.2 | 12.8 ± 3.6 | 0.03 | |
| Urinary magnesium excretion (mmol/day) | 4.0 ± 2.1 | 4.1 ± 2.1 | 4.4 ± 2.3 | 3.3 ± 1.7 *,† | 3.2 ± 1.4 *,† | 0.001 | |
| Urinary phosphate excretion (mmol/day) | 27.5 ± 11.6 | 28.2 ± 12.2 | 30.3 ± 12.6 | 22.7 ± 7.7 *,† | 25.0 ± 7.9 | 0.001 | |
| Sodium-to-potassium ratio (mmol/mmol) | 2.5 ± 1.0 | 2.5 ± 1.0 | 2.8 ± 1.2 | 2.2 ± 0.7 † | 2.3 ± 0.8 | 0.004 | |
| Calculated intake | |||||||
| Dietary salt intake (g/day) | 10.9 ± 4.7 | 11.0 ± 4.3 | 12.7 ± 5.6 * | 8.7 ± 4.0 *,† | 9.7 ± 3.9 † | <0.001 | |
| Salt intake ≤6 g/day | 53 (12) | 26 (10) | 5 (6) | 15 (29) | 7 (14) | <0.001 | |
| Dietary potassium intake (g/day) | 3.9 ± 1.3 | 4.0 ± 1.4 | 4.1 ± 1.1 | 3.5 ± 1.3 | 3.6 ± 0.9 | 0.01 | |
| Potassium intake ≥3.5 g/day | 290 (66) | 173 (69) | 62 (73) | 27 (53) | 29 (59) | 0.06 | |
| Dietary protein intake (g/day) | 92 ± 27 | 94 ± 28 | 98 ± 29 | 80 ± 23 *,† | 84 ± 21 † | 0.001 | |
| Variable | BP On Target | BP Not On Target | p-Value | |
|---|---|---|---|---|
| Patient characteristics | n = 239 | n = 210 | ||
| Age (years) | 63 ± 9 | 63 ± 9 | 0.36 | |
| Male, n (%) | 126 (53) | 134 (64) | 0.02 | |
| Years T2DM (years) | 11 (7–17) | 12 (7–18) | 0.26 | |
| Serum HbA1C (mmol/mol) | 56 ± 11 | 59 ± 12 | 0.03 | |
| Insulin use, n (%) | 149 (62) | 136 (65) | 0.60 | |
| Systolic blood pressure (mmHg) | 125 ± 10 | 149 ± 13 | <0.001 | |
| Diastolic blood pressure (mmHg) | 70 ± 8 | 80 ± 9 | <0.001 | |
| eGFR <60, n (%) | 53 (22) | 51 (24) | 0.60 | |
| Albuminuria, n (%) | 40 (17) | 95 (46) | <0.001 | |
| Pharmacological management | ||||
| RAASi, n (%) | 163 (68) | 134 (64) | 0.33 | |
| β-blockers, n (%) | 115 (48) | 93 (44) | 0.42 | |
| Thiazide diuretics, n (%) | 71 (30) | 66 (31) | 0.69 | |
| Calcium antagonists, n (%) | 50 (21) | 52 (25) | 0.33 | |
| Loop diuretics, n (%) | 52 (22) | 29 (14) | 0.03 | |
| Potassium sparing diuretics, n (%) | 22 (9) | 21 (10) | 0.78 | |
| Number of antihypertensives | 2 (1–3) | 2 (1–3) | 0.51 | |
| No antihypertensive therapy, n (%) | 39 (16) | 44 (21) | 0.85 | |
| 1 drug, n (%) | 47 (20) | 42 (20) | ||
| 2 drugs, n (%) | 64 (27) | 45 (21) | ||
| 3 drugs, n (%) | 50 (21) | 43 (21) | ||
| 4 drugs, n (%) | 29 (12) | 27 (13) | ||
| 5+ drugs, n (%) | 10 (4) | 9 (4) | ||
| Hypertension requiring 4+ drugs, n (%) | 39 (16) | 79 (38) | <0.001 | |
| Total number of drugs | 7.0 ± 2.6 | 6.7 ± 2.8 | 0.30 | |
| Non-pharmacological management | ||||
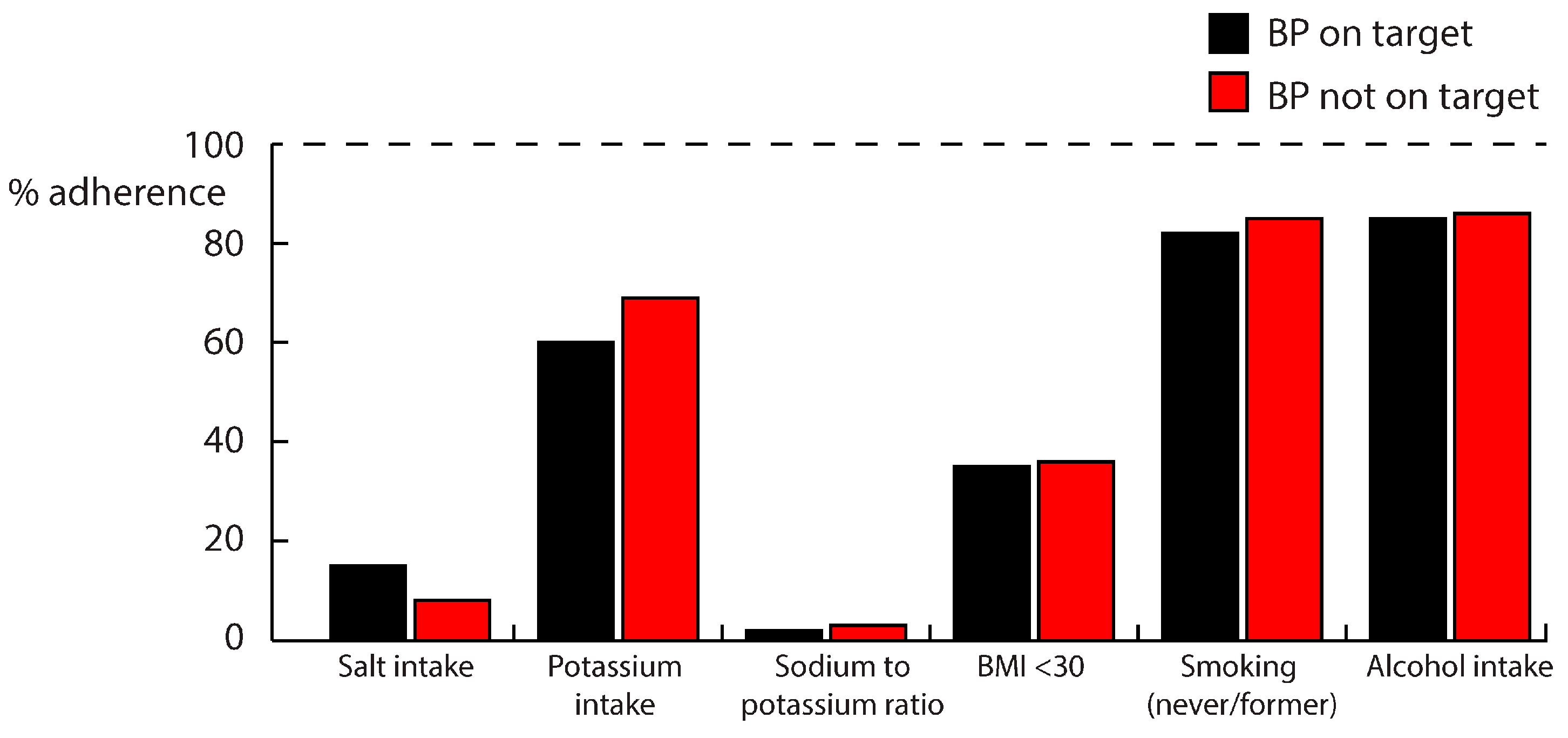
| BMI (kg/m2) | 32.8 ± 5.8 | 32.9 ± 6.7 | 0.89 | |
| Serum 25 (OH) Vitamin D (nmol/L) | 43 ± 20 | 41 ± 20 | 0.22 | |
| Urinary excretion | ||||
| Urinary creatinine excretion (mmol/day) | 13.6 ± 4.9 | 14.1 ± 4.7 | 0.22 | |
| Urinary magnesium excretion (mmol/day) | 3.9 ± 2.1 | 4.0 ± 1.9 | 0.43 | |
| Urinary phosphate excretion (mmol/day) | 26.9 ± 12.3 | 28.2 ± 10.7 | 0.26 | |
| Sodium-to-potassium ratio (mmol/mmol) | 2.5 ± 1.0 | 2.5 ± 0.9 | 0.49 | |
| Calculated intake | ||||
| Dietary salt intake (g/day) | 10.7 ± 4.8 | 11.1 ± 4.4 | 0.47 | |
| Dietary potassium intake (g/day) | 3.8 ± 1.3 | 4.0 ± 1.2 | 0.15 | |
| Dietary protein intake (g/day) | 90 ± 29 | 93 ± 26 | 0.29 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gant, C.M.; Binnenmars, S.H.; Berg, E.V.d.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients 2017, 9, 709. https://doi.org/10.3390/nu9070709
Gant CM, Binnenmars SH, Berg EVd, Bakker SJL, Navis G, Laverman GD. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients. 2017; 9(7):709. https://doi.org/10.3390/nu9070709
Chicago/Turabian StyleGant, Christina M., S. Heleen Binnenmars, Else Van den Berg, Stephan J. L. Bakker, Gerjan Navis, and Gozewijn D. Laverman. 2017. "Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT)" Nutrients 9, no. 7: 709. https://doi.org/10.3390/nu9070709





