An Increased Dietary Supply of Medium-Chain Fatty Acids during Early Weaning in Rodents Prevents Excessive Fat Accumulation in Adulthood
Abstract
:1. Introduction
2. Methods and Materials
2.1. Rationale
2.2. Animals
2.3. Experimental Diets
2.4. Mouse Study
2.5. Rat Study
2.6. Termination and Dissection
2.7. Cytological and Biochemical Analyses of Mouse and Rat WAT
2.8. Plasma Analyses
2.9. Statistical Analyses
3. Results
3.1. Effects of Early Diet on Milk and Pup Erythrocyte FA Composition
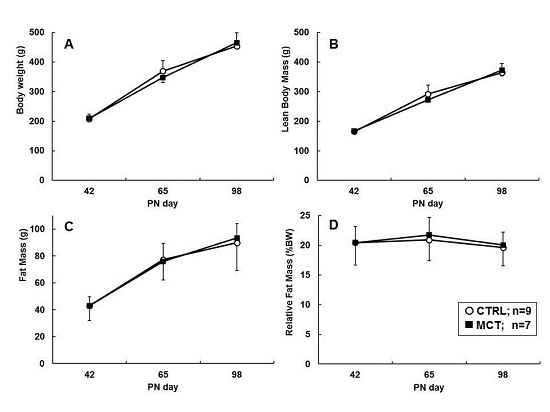
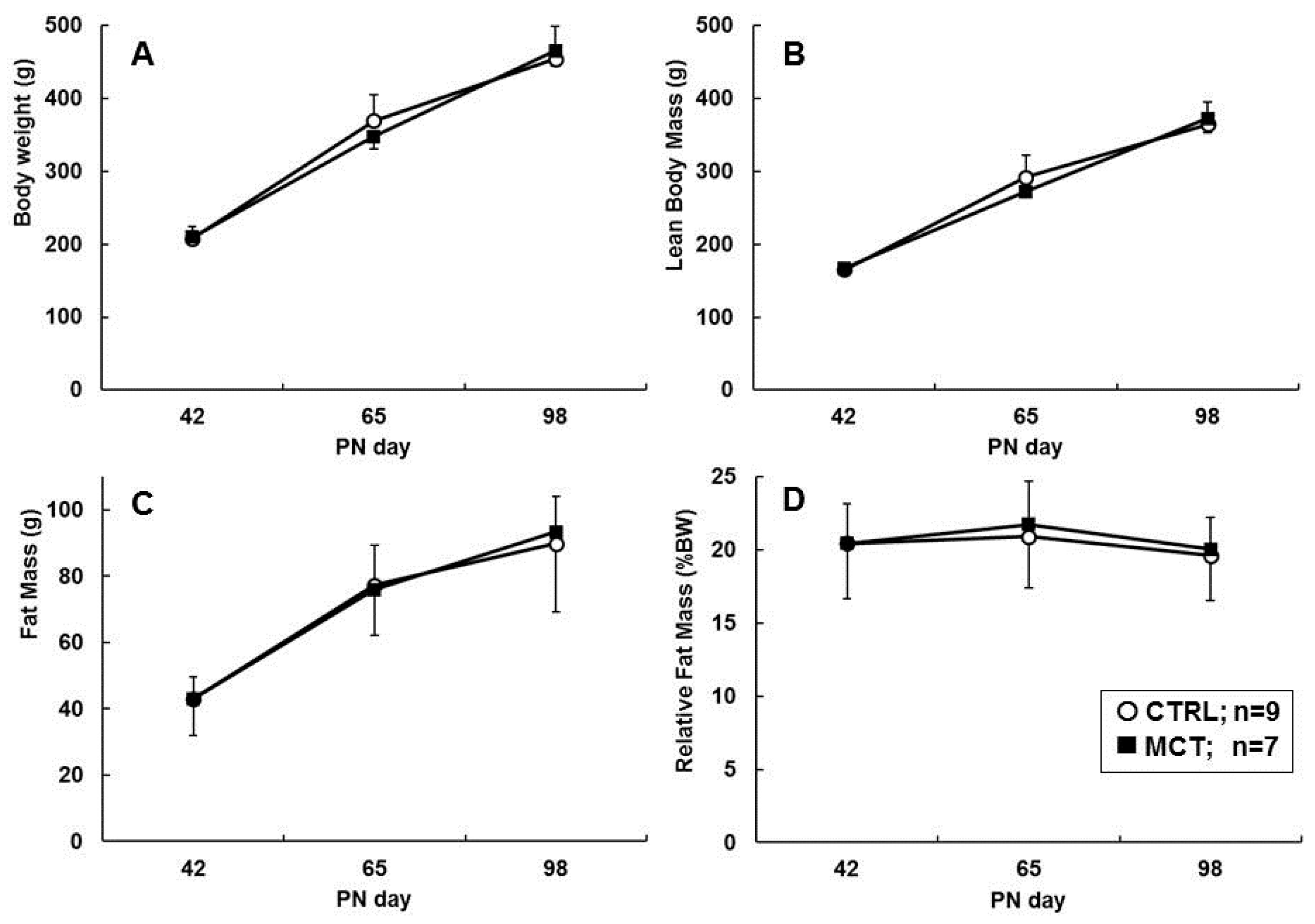
3.2. Direct Effects of Early Diet on Growth, Body Composition, and Food Intake
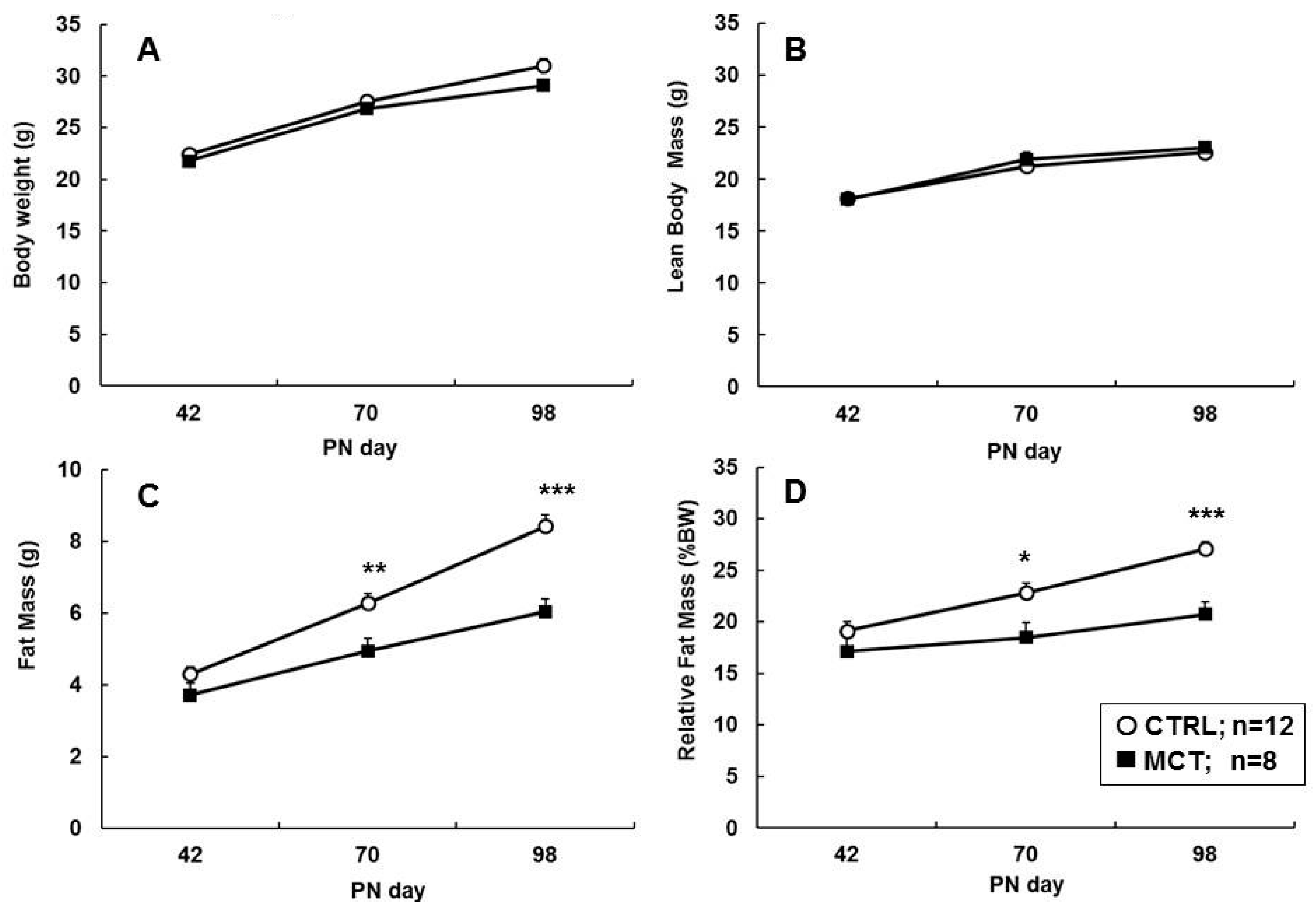
3.3. Effects of Early Diet on Food Intake and Body Composition during WSD Challenge
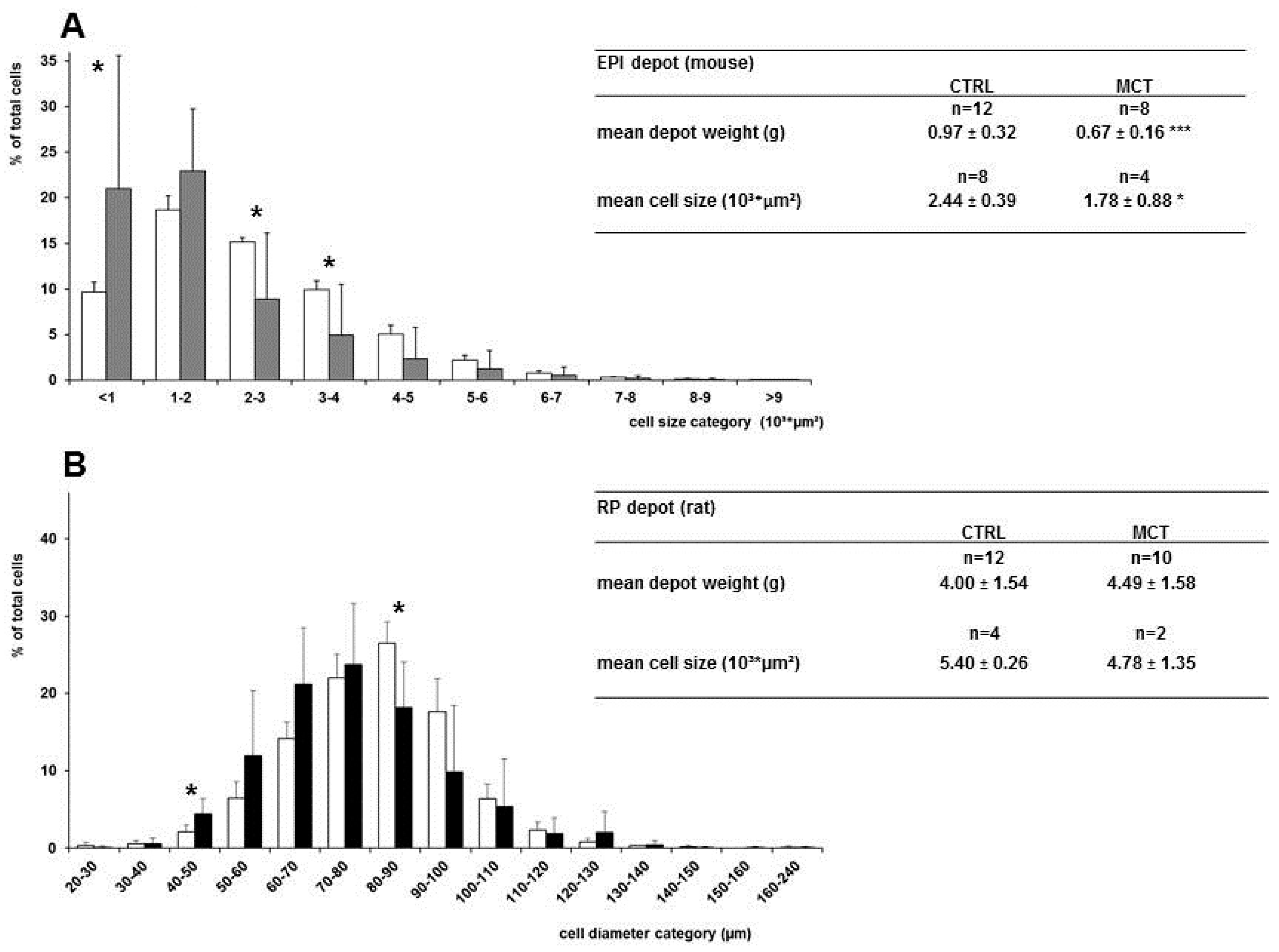
3.4. Effects of Early Diet on Adipocyte Size Distribution after WSD Challenge
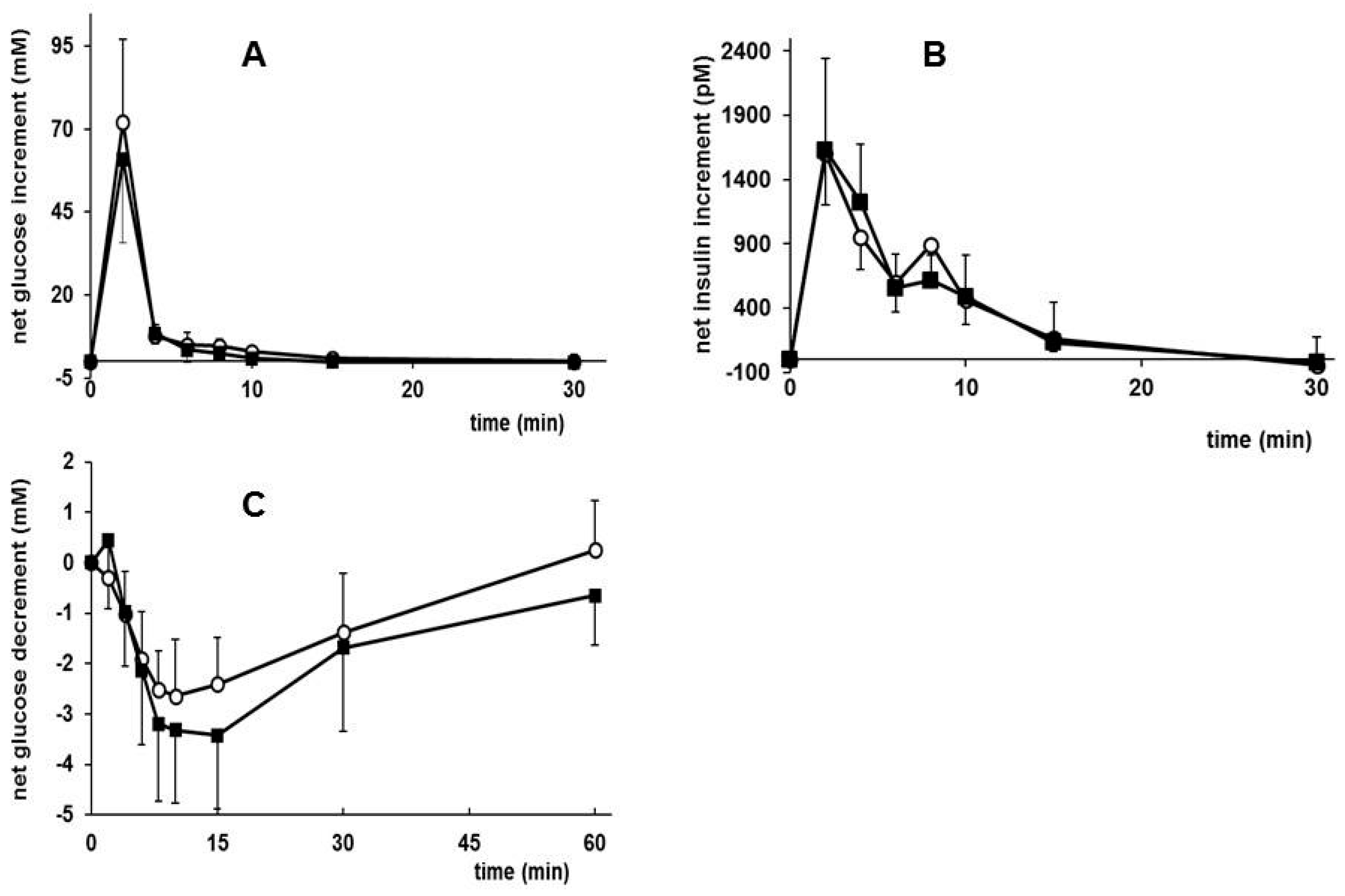
3.5. Effects of Early Diet on Metabolic Plasma Parameters after WSD Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Symonds, M.; Sebert, S.; Hyatt, M.; Budge, H. Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Brunner, S. Early fatty acid exposure and later obesity risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Painter, R.; Ocke, M.; Buller, H.; Bossuyt, P.; Roseboom, T. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am. J. Clin. Nutr. 2008, 88, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Kennedy, K.; Lanigan, J.; Fewtrell, M.; Cole, T.; Stephenson, T. Nutrition in infancy and long-term risk of obesity: Evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Ross, M. Fetal programming of adipose tissue: Effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin. Reprod. Med. 2011, 29, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G.; Guesnet, P. Fatty acid composition of fat is an early determinant of childhood obesity: A short review and an opinion. Obes. Rev. 2004, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.; Cook-Johnson, R.; James, M.; Miljkovic, D.; Duthoit, E.; Gibson, R. Opposing effects of omega-3 and omega-6 long chain polyunsaturated fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J. Nutr. Metab. 2010, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R. Increases in morbid obesity in the USA: 2000–2005. Public Health 2007, 121, 492–496. [Google Scholar] [CrossRef] [PubMed]
- McAllister, E.; Dhurandhar, N.; Keith, S.; Aronne, I.; Barger, J.; Baskin, M. Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr. 2009, 49, 868–913. [Google Scholar] [CrossRef] [PubMed]
- Odle, J. New insights into the utilization of medium-chain triglycerides by the neonate: Observations from a pigplet model. J. Nutr. 1997, 127, 1061–1067. [Google Scholar] [PubMed]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Bosarge, A.; Goree, L.; Darnell, B. Medium chain triglyceride oil consumption as part of a weight loss diet does not lead to an adverse metabolic profile when compared to olive oil. J. Am. Coll. Nutr. 2008, 27, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Jones, P. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [PubMed]
- Lemarié, F.; Beauchamp, E.; Legrand, P.; Rioux, V. Revisiting the metabolism and physiological functions of caprylic acid (C8:0) with special focus on ghrelin octanoylation. Biochemie 2016, 120, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Siemelink, M.; Verhoef, A.; Dormans, J.; Span, P.; Piersma, A. Dietary fatty acid composition during pregnancy and lactation in the rat programs growth and glucose metabolism in the offspring. Diabetologica 2002, 45, 1397–1403. [Google Scholar]
- Samuelsson, A.; Matthews, P.; Argenton, M.; Christie, M.; McDonnell, J.; Jansen, E. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; McGuire, J.; Cheema, S. Developmental programming of lipid metabolism and aortic vascular function in C57BL/6 mice: A novel study suggesting an involvement of LDL-receptor. Am. J. Physiol. 2009, 296, R1029–R1040. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, V.; Wickman, A.; Strandvik, B.; Gabrielsson, B.; Olsson, B. Prenatal essential fatty acid deficiency in mice results in long-term gender-specific effects on body weight and glucose metabolism. Mol. Med. Rep. 2011, 4, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.; Miljkovic, D.; Fong, L.; Xian, C.; Duthoit, E.; Gibson, R. Maternal omega-3 supplementation increases fat mass in male and female rat offspring. Front. Genet. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.; Hirsch, J. Postnatal development of adipocyte cellularity in the normal rat. J. Lipid Res. 1974, 15, 474–483. [Google Scholar] [PubMed]
- Burdge, G.; Lillycrop, K.; Phillips, E.; Slater-Jefferies, J.; Jackson, A.; Hanson, M.A. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J. Nutr. 2009, 139, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.; Sigal, R.; Goldfield, G.; Prud’homme, D.; Kenny, G. Overweight and obese teenagers: Why is adolescence a critical period? Pediatr. Obes. 2012, 7, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Oosting, A.; Kegler, D.; Böhm, G.; Jansen, H.; van de Heijning, B.; van der Beek, E. N-3 long-chain polyunsaturated fatty acids prevent excessive fat deposition in adulthood in a mouse model of postnatal nutritional programming. Pediatr. Res. 2010, 68, 494. [Google Scholar] [CrossRef] [PubMed]
- Oosting, A.; Kegler, D.; van de Heijning, B.; Verkade, H.; van der Beek, E. Reduced linoleic acid intake in early postnatal life improves metabolic outcomes in adult rodents following a western-style diet challenge. Nutr. Res. 2015, 35, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.; Nielsen, F.; Fahey, G. AIN-93 purified diets for laboratory rodents: Final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Sengupta, P. A scientific review of age determination for a laboratory rat: How old is it in comparison with human age? Biomed. Int. 2011, 2, 81–89. [Google Scholar]
- Oosting, A.; Verkade, H.; Kegler, D.; van de Heijning, B.; van der Beek, E. Rapid and selective manipulation of milk fatty acid composition in mice through the maternal diet during lactation. J. Nutr. Sci. 2015, 4, e19. [Google Scholar] [CrossRef] [PubMed]
- Levant, B.; Ozias, M.; Carlson, S. Diet (n-3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. J. Nutr. 2007, 137, 2425–2430. [Google Scholar] [PubMed]
- DiGirolamo, M.; Fine, J. Cellularity measurements. Methods Mol. Biol. 2001, 155, 65–75. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Lee, S.; Muniyappa, R.; Yan, X.; Chen, H.; Yue, L.; Hong, E. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am. J. Physiol. 2008, 294, E261–E270. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Chen, H.; Muzumdar, R.; Einstein, F.; Yan, X. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic clamp estimates in rats. Am. J. Physiol. 2009, 297, E1023–E1029. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.; Garza, C. Early postnatal nutrition determines adult pancreatic glucose-responsive insulin secretion and islet gene expression in rats. J. Nutr. 2002, 132, 357–364. [Google Scholar]
- Oosting, A.; Kegler, D.; Wopereis, H.; Teller, I.; van de Heijning, B.; Verkade, H.; van der Beek, E. Size and phospholipid coating of lipid droplets in the diet of young mice modify body fat accumulation in adulthood. Pediatr. Res. 2012, 72, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Paschos, G.; Ibrahim, S.; Song, W. Obesity in mice with adipocyte-specific deletion of clock component arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.; Bouyer, K.; Oosting, A.; Simerly, R.B.; van der Beek, E.M. Postnatal dietary fatty acid composition permanently affects the structure of hypothalamic pathways controlling energy balance in mice. Am. J. Clin. Nutr. 2013, 98, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.M.; Li, Y.; Ning, H.; Wang, C.; Liu, J.R.; Sun, C.H. High dietary intake of medium-chain fatty acids during pregnancy in rats prevents later-life obesity in their offspring. J. Nutr. Biochem. 2010, 22, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.; Arner, E.; Westermark, P. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.; Matthew, W. Growth and lipolysis of rat adipose tissue: Effect of age, body weight, and food intake. J. Lipid Res. 1971, 12, 286–293. [Google Scholar] [PubMed]
- Gruen, R.; Kava, R.; Greenwood, M. Development of basal lipolysis and fat cell size in the epididymal fat pad of normal rats. Metabolism 1980, 29, 246–253. [Google Scholar] [CrossRef]
- Carnielli, V.; Rossi, K.; Badon, T.; Gregori, B.; Verlato, G.; Orzali, A.; Zachello, F. Medium-chain triacylglycerols in formulas for preterm infants: Effecy on plasma lipids, circulating concentrations of medium-chain fatty acids, and essential fatty acids. Am. J. Clin. Nutr. 1996, 64, 152–158. [Google Scholar] [PubMed]
- Madsen, L.; Petersen, R.; Kristiansen, K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta 2005, 1740, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G.; Massiera, F.; Weill, P.; Legrand, P.; Alessandri, J.; Guesnet, P. Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 2006, 45, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.; Gibson, R.; Makrides, M. Effect of long-chain polyunsaturated fatty acid supplementation during pregnancy or lactation on infant and child body composition: A systematic review. Am. J. Clin. Nutr. 2010, 92, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.; Gibson, R.; Makrides, M. The effect of maternal omega-3 long-chain polyunsaturated fatty acid (n-3 LCPUFA) supplementation during pregnancy and/or lactation on body fat mass in the offspring: A systematic review of animal studies. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Lauritzen, L.; Brasholt, M.; Buhl, T.; Bisgaard, H. Polyunsaturated fatty acid content of mother’s milk is associated with childhood body composition. Pediatr. Res. 2012, 72, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.; Harvey, N.; Robinson, S.; Ntani, G.; Davies, J.; Inskip, H. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Brunner, S.; Amann-Gassner, U. The role of dietary fatty acids for early human adipose tissue growth. Am. J. Clin. Nutr. 2013, 98, 549S–555S. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K. Important differences exist in the dose-response relationship between diet and immune cell fatty acids in humans and rodents. Lipids 2007, 42, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jou, W.; Gavrilova, O.; Hall, K. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS ONE 2009, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Schothorst, E.; Bunschoten, A.; Verlinde, E.; Schrauwen, P.; Keijer, J. Glycemic index differences of high-fat diets modulate primarily lipid metabolism in murine adipose tissue. Physiol. Genom. 2011, 43, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Funke, S.; Fink, M.; Demmelmair, H.; Turini, M.; Crozier, G.; Koletzko, B. Plasma fatty acids and [13C] linoleic acid metabolism in preterm infants fed a formula with medium-chain triglycerides. J. Lipid Res. 2003, 44, 41–48. [Google Scholar] [CrossRef] [PubMed]
| (%FA) | Early Diet | WSD | ||
|---|---|---|---|---|
| CTRL 1 | MCT | |||
| C-8:0 | Caprylic acid | 1.65 | 15.20 | 1.06 |
| C-10:0 | Capric acid | 1.52 | 3.54 | 0.87 |
| C-12:0 | Lauric acid | 10.83 | 1.59 | 6.02 |
| C-14:0 | Myristic acid | 4.45 | 1.13 | 4.17 |
| C-16:0 | Palmitic acid | 18.73 | 14.67 | 23.37 |
| C-16:1 n-7 | Palmitoleic acid | 0.15 | 0.15 | 1.2 |
| C-18:0 | Stearic acid | 3.61 | 2.84 | 13.66 |
| C-18:1 n-9 | Oleic acid | 38.16 | 39.84 | 36.22 |
| C-18:2 n-6 | Linoleic acid (LA) | 15.26 | 15.15 | 8.80 |
| C-18:3 n-3 | Alpha linolenic acid (ALA) | 2.96 | 2.97 | 1.67 |
| C-20:0 | Arachidic acid | 0.38 | 0.37 | 0.28 |
| C-20:1 n-9 | Eicosaenoic acid | 0.43 | 0.48 | 0.27 |
| C-20:4 n-6 | Arachidonic acid | 0 | 0 | 0 |
| C-20:5 n-3 | Eicosapentaenoic acid (EPA) | 0 | 0 | 0 |
| C-22:0 | Behenic acid | 0.32 | 0.32 | 0.09 |
| C-22:1 n-9 | Erucic acid | 0 | 0 | 0 |
| C-22:5 n-3 | Docosapentaenoic acid | 0 | 0 | 0 |
| Σ MCFA (C8:C12) | 14.00 | 20.34 | 7.95 | |
| Σ SFA % | 41.58 | 39.79 | 50.09 | |
| Σ MUFA % | 38.74 | 40.51 | 37.70 | |
| Σ PUFA % | 18.22 | 18.20 | 10.47 | |
| Σ n-6 | 15.26 | 15.23 | 8.80 | |
| Σ n-3 | 2.96 | 2.97 | 1.67 | |
| n-6/n-3 ratio | 5.16 | 5.12 | 5.26 | |
| LA/ALA ratio | 5.16 | 5.10 | 5.26 | |
| (g/Day) | Mice | Rats | ||
|---|---|---|---|---|
| CTRL | MCT | CTRL | MCT | |
| Early diet | 3.18 ± 0.26 (n = 6) | 3.15 ± 0.40 (n = 4) | 21.6 ± 4.5 (n = 3) | 19.1 ± 0.8 (n = 3) |
| WSD | 2.98 ± 0.35 (n = 6) | 3.05 ± 0.51 (n = 4) | 21.1 ± 4.8 (n = 12) | 22.9 ± 2.1 (n = 10) |
| Mouse | Rat | ||||
|---|---|---|---|---|---|
| CTRL | MCT | CTRL | MCT | ||
| Fat depots | EPI (g) | 0.97 ± 0.32 | 0.61 ± 0.17 *** | 6.47 ± 1.78 | 6.75 ± 1.95 |
| PR (g) | 0.11 ± 0.04 | 0.09 ± 0.05 # | 1.00 ± 0.29 | 1.18 ± 0.15 | |
| RP (g) | - | - | 4.00 ± 1.54 | 4.49 ± 1.58 | |
| ING (g) | 0.64 ± 0.15 | 0.33 ± 0.15 *** | 5.72 ± 1.75 | 5.94 ± 1.88 | |
| Liver (g) | 1.32 ± 0.21 | 1.18 ± 0.20 # | 15.72 ± 2.87 | 15.42 ± 2.12 | |
| TG (w%) | 13.87 ± 7.05 | 7.13 ± 3.53 ** | - | - | |
| mg TG/mg protein | 0.30 ± 0.16 | 0.18 ± 0.08 ** | - | - | |
| Pancreas (mg) | 92 ± 51 | 142 ± 14 * | 1178 ± 231 | 1200 ± 166 | |
| Brain (mg) | 452 ± 13 | 454 ± 18 | 1999 ± 122 | 1990 ± 79 | |
| Kidneys (mg) | 350 ± 31 | 337 ± 125 | - | - | |
| Thymus (mg) | 56 ± 6 | 48 ± 7 | - | - | |
| CTRL | MCT | |||
|---|---|---|---|---|
| Plasma | Adipokines | Adiponectin (mg/L) | 8.64 ± 0.64 | 8.67 ± 1.09 |
| Leptin (µg/L) | 4.97 ± 2.52 | 1.89 ± 1.01 ** | ||
| Resistin (µg/L) | 4.06 ± 0.83 | 4.00 ± 1.24 | ||
| MCP-1 (ng/L) | 9.56 ± 4.24 | 10.26 ± 6.81 | ||
| tPAI-1 (µg/L) | 1.84 ± 0.56 | 2.19 ± 0.36 | ||
| TNFα (ng/L) | 4.26 ± 0.46 | 4.60 ± 0.33 | ||
| IL-6 (ng/L) | 6.36 ± 2.74 | 7.78 ± 5.78 | ||
| Insulin sensitivity | Glucose (mM) | 16.51 ± 2.35 | 13.66 ± 5.07 # | |
| Insulin (pM) | 114.62 ± 87.27 | 64.32 ± 38.03 # | ||
| HOMA-IR | 89.11 ± 75.79 | 41.72 ± 26.34 # | ||
| Lipids | TG (mg/L) | 2.40 ± 0.70 | 2.60 ± 0.95 | |
| TC (mg/L) | 1262 ± 183 | 1017 ± 355 * | ||
| FFA (mM) | 0.50 ± 0.19 | 0.44 ± 0.09 | ||
| EPI Fat Depot | DNA (ng/mg WAT) | 32.3 ± 8.9 | 45.1 ± 19.4 * | |
| TG (mg/mg WAT) | 0.84 ± 0.9 | 0.83 ± 0.9 | ||
| TG/DNA | 28.7 ± 10.4 | 20.5 ± 6.2 * | ||
| Total DNA (µg) | 28.9 ± 4.1 | 25.8 ± 4.4 # |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van de Heijning, B.J.M.; Oosting, A.; Kegler, D.; Van der Beek, E.M. An Increased Dietary Supply of Medium-Chain Fatty Acids during Early Weaning in Rodents Prevents Excessive Fat Accumulation in Adulthood. Nutrients 2017, 9, 631. https://doi.org/10.3390/nu9060631
Van de Heijning BJM, Oosting A, Kegler D, Van der Beek EM. An Increased Dietary Supply of Medium-Chain Fatty Acids during Early Weaning in Rodents Prevents Excessive Fat Accumulation in Adulthood. Nutrients. 2017; 9(6):631. https://doi.org/10.3390/nu9060631
Chicago/Turabian StyleVan de Heijning, Bert J. M., Annemarie Oosting, Diane Kegler, and Eline M. Van der Beek. 2017. "An Increased Dietary Supply of Medium-Chain Fatty Acids during Early Weaning in Rodents Prevents Excessive Fat Accumulation in Adulthood" Nutrients 9, no. 6: 631. https://doi.org/10.3390/nu9060631