Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Strawberry Extract
2.2. Strawberry Extract Characterization
2.2.1. Total Phenolic Compounds (TPC) and Flavonoids Content Determination
2.2.2. Vitamin C Content Determination
2.2.3. Identification and Quantification of Strawberries Anthocyanins
2.2.4. Total Antioxidant Capacity (TAC) Determination
2.3. Cells Culture and Cells’ Lysates Preparation
2.4. Cytotoxicity Assay
2.5. Western Blotting Analysis
2.6. Mitochondrial Functionality
2.7. Determination of Total Cholesterol, LDL-Cholesterol, and Triglycerides Levels
2.8. Determination of Total Lipid Accumulation by Oil Red O Staining
2.9. Statistical Analysis
3. Results
3.1. Characterization of Romina Strawberry Extract
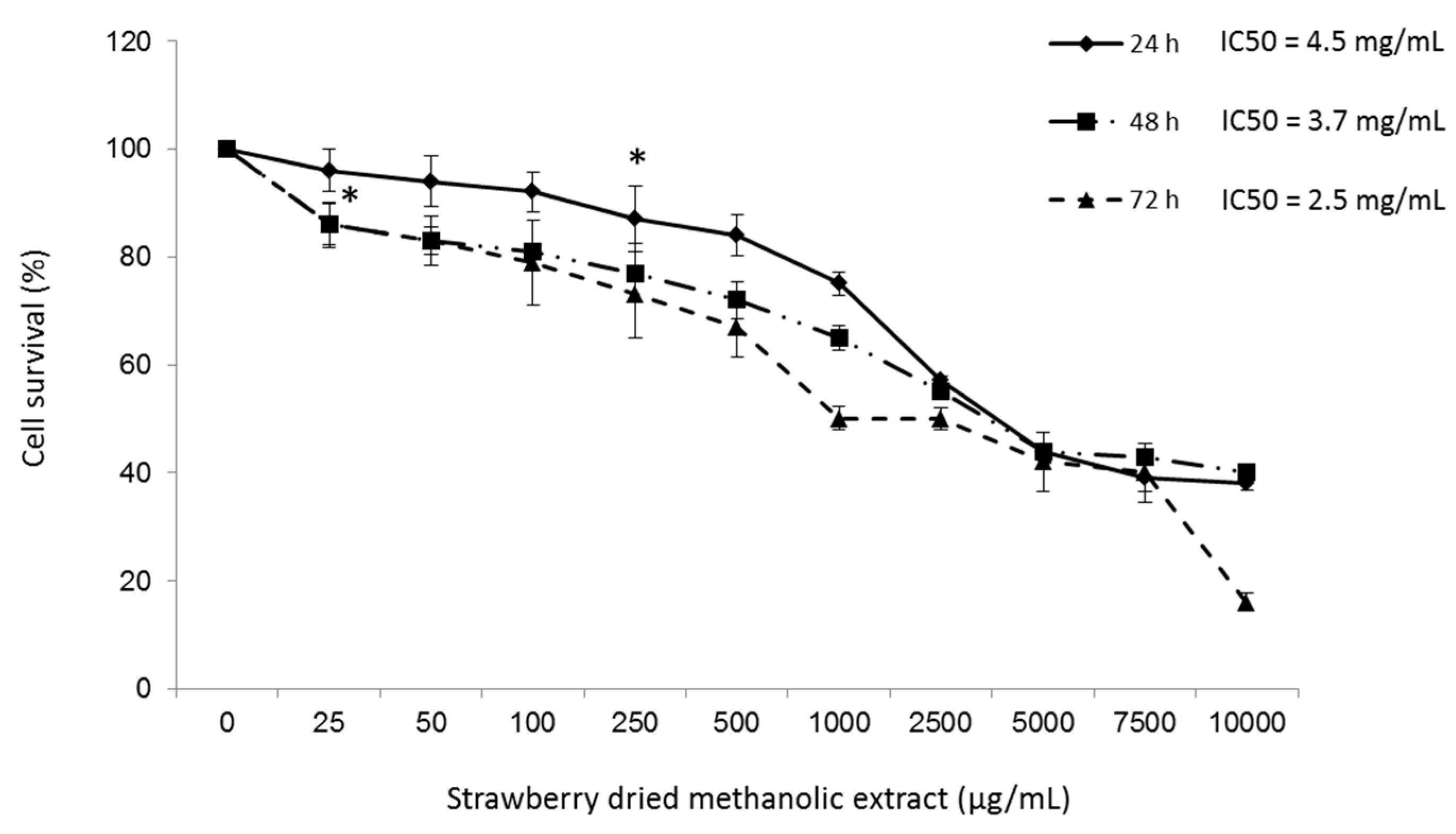
3.2. Effects of Strawberry Extract on HepG2 Cell Viability
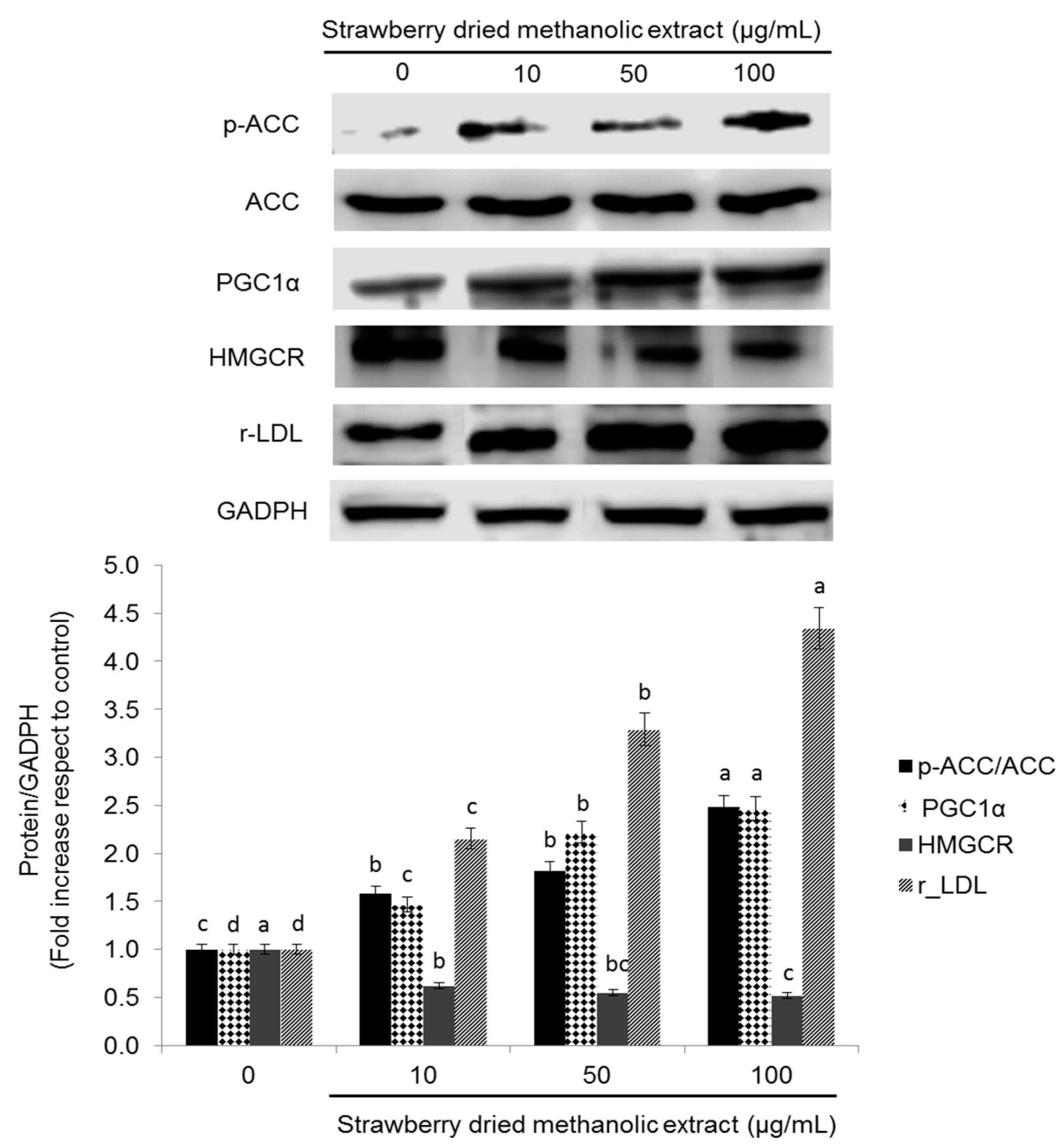
3.3. Effects of Strawberry Extract on AMPK Activation
3.4. Effects of Strawberry Extract on Lipid Profile
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldberg, I.J. Clinical review 124: Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S.; Mace, M.D.; Smith, D.A.; Mehta, A.E.; Ganda, O.; Handelsman, Y.; Rodbard, H.W.; Shepherd, M.D.; Seibel, J.A. American association of clinical endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr. Pract. 2012, 18 (Suppl. 1), 1–78. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. New concepts in cardiovascular disease. J. Restor. Med. 2013, 2, 30–44. [Google Scholar] [CrossRef]
- Wagner, K.H.; Brath, H. A global view on the development of non-communicable diseases. Prev. Med. 2012, 54, 38–41. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Non-Communicable Diseases 2014; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Wanner, C.; Quaschning, T. Dyslipidemia and renal disease: Pathogenesis and clinical consequences. Curr. Opin. Nephrol. Hypertens. 2001, 10, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Giampieri, F.; Alvarez Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Bullon, P.; Battino, M. AMPK as a New Attractive Therapeutic Target for Disease Prevention: The Role of Dietary Compounds. Curr. Drug Targets 2016, 17, 865–889. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, L.; Perez-Lopez, P.; Giampieri, F.; Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Mezzetti, B.; Battino, M. The genetic aspects of berries: From field to Health. J. Sci. Food Agric. 2015, 96, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Xu, F.D.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Torronen, R.; Sarkkinen, E.; Niskanen, E.; Tapola, N.; Kilpi, K.; Niskanen, L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2012, 107, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int. J. Biol. Macromol. 2007, 41, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Alvarez-Suarez, J.M.; Busco, F.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry consumption improves plasma antioxidant status and erythrocyte resistance to oxidative haemolysis in humans. Food Chem. 2011, 128, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Parelman, M.A.; Storms, D.H.; Kirschke, C.P.; Huang, L.; Zunino, S.J. Dietary strawberry powder reduces blood glucose concentrations in obese and lean C57BL/6 mice, and selectively lowers plasma C-reactive protein in lean mice. Br. J. Nutr. 2012, 108, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, B. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxides radicals. Food Chem. 1998, 64, 555–559. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional values of tomatoes by increasing the total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Helsper, J.P.; de Vos, C.H.; Maas, F.M.; Jonker, H.H.; van den Broeck, H.C.; Jordi, W.; Pot, C.S.; Keizer, L.C.; Schapendonk, A.H. Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol. Plant. 2003, 117, 171–187. [Google Scholar] [CrossRef]
- Terefe, N.S.; Kleintschek, T.; Gamage, T.; Fanning, K.J.; Netzel, G.; Versteeg, C.; Netzel, M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov. Food Sci. Emerg. Technol. 2013, 19, 57–65. [Google Scholar] [CrossRef]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Activity-guided isolation and identification of free radical scavenging components from an aqueous extract of Coleus aromaticus. Food Chem. 2007, 100, 356–361. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Srivattana, A.; Bunyapraphatsara, N.; Puthong, S.; Laohathai, K. Cytotoxicity assay of hispidulin and quercetin using colorimetric technique. Mahidol Univ. J. Pharm. Sci. 1991, 18, 25–31. [Google Scholar]
- Studzinski, G.P. (Ed.) Cell Growth and Apoptosis a Practical Approach; Oxford University Press: Oxford, UK, 1995; ISBN 9780199635696. [Google Scholar]
- Liu, J.F.; Ma, Y.; Wang, Y.; Du, Z.Y.; Shen, J.K.; Peng, H.L. Reduction of lipid accumulation in HepG2 Cells by luteolin is associated with activation of AMPK and Mitigation of oxidative stress. Phytother. Res. 2010, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Capocasa, F.; Scalzo, J.; Mezzetti, B.; Battino, M. Combining quality and antioxidant attributes in the strawberry: The role of genotype. Food Chem. 2008, 111, 872–878. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, J.; Mezzetti, B.; Giampieri, F.; Alvarez-Suarez, J.M.; Quiles, J.L.; Gonzalez-Alonso, A.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Gonzalez Paramas, A.M.; Santos-Buelga, C.; et al. Doxorubicin-Induced Oxidative Stress in Rats Is Efficiently Counteracted by Dietary Anthocyanin Differently Enriched Strawberry (Fragaria × ananassa Duch.). J. Agric. Food Chem. 2014, 62, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Schimmack, G.; De Fronzo, R.; Musi, N. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes. Obes. Metab. 2006, 8, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, W.; Miner, J.; Fromm, M. Cross regulation of Sirtuin 1, AMPK and PPARγ in conjugated linoleic acid treated adipocytes. PLoS ONE 2012, 7, e48874. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Niknejad, I.; Gorn-Hondermann, I.; Dayekh, K.; Dimitroulakos, J. Lovastatin Induces Multiple Stress Pathways Including LKB1/AMPK Activation That Regulate Its Cytotoxic Effects in Squamous Cell Carcinoma Cells. PLoS ONE 2012, 7, e46055. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lee, T.S.; Zhu, M.; Gu, C.; Wang, Y.; Zhu, Y.; Shyy, J.Y. Statins Activate AMP-Activated Protein Kinase In Vitro and In Vivo. Circulation 2006, 114, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, L.; Shyy, J.Y.; Sun, W. Atorvastatin Activates CaMKK-beta as an Upstream Kinase of AMPK in Endothelium. Circulation 2008, 118, S_404. [Google Scholar]
- Choi, H.C.; Song, P.; Xie, Z.; Wu, Y.; Xu, J.; Zhang, M.; Dong, Y.; Wangs, L.K.; Zou, M.H. Reactive Nitrogen Species Is Required for the Activation of the AMP-activated Protein Kinase by Statin In Vivo. J. Biol. Chem. 2008, 283, 20186–20197. [Google Scholar] [CrossRef] [PubMed]
- Hakkinen, S.H.; Torronen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Hannun, S.M. Potential impact of strawberries on human health: A review of the science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The Healthy Effects of Strawberry Polyphenols: Which Strategy behind Antioxidant Capacity? Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S46–S59. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Li, X. Sirtuin 1 in lipid metabolism and obesity. Ann. Med. 2011, 43, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Vanhoutte, P.M. SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Lett. 2011, 585, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Lee, S.M.; Shin, S.M.; Hwang, S.J.; Brooks, J.S.; Kang, H.E.; Lee, M.G.; Kim, S.C.; Kim, S.G. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic. Biol. Med. 2009, 47, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Honokiol activates the LKB1–AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol. Appl. Pharmacol. 2015, 284, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.; Handschin, C.; St Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.G.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. The LDL Receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Desler, C.; Hansen, T.L.; Frederiksen, J.B.; Marcker, M.L.; Singh, K.K.; Rasmussen, L.J. Is There a Link between Mitochondrial Reserve Respiratory Capacity and Aging? J. Aging. Res. 2012, 2012, 192503:1–192503:9. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, Y.; Sun, T.Q.; Markovtsov, V.; Foretz, M.; Li, W.; Nguyen, H.; Li, Y.; Pan, A.; Uy, G.; Gross, L.; et al. AMPK activation through mitochondrial regulation results in increaed substrated oxidation and improved metabolic parmeter in models of diabetes. PLoS ONE 2013, 8, e81870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, J. Mitochondrial inhibitor as a new class of insulin sensitizer. Acta Pharm. Sin. B 2012, 2, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Colca, J.R.; Vander Lugt, J.T.; Adams, W.J.; Shashlo, A.; McDonald, W.G. Clinical proof-of-concept study with MSDC-0160, a prototype mTOT-modulating insulin sensitizer. Clin. Pharmacol. Ther. 2013, 93, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Gadalla, A.E.; Olsen, G.S.; Hardie, D.G. The Antidiabetic Drug Metformin Activates the AMP-Activated Protein Kinase Cascade via an Adenine Nucleotide-Independent Mechanism. Diabetes 2002, 51, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Brunmair, B.; Staniek, K.; Gras, F.; Scharf, N.; Althaym, A.; Clara, R.; Roden, M.; Gnaiger, E.; Nohl, H.; Waldhausl, W.; et al. Thiazolidinediones, like metformin, inhibit respiratory complex I. A common mechanism contributing to their antidiabetic action? Diabetes 2004, 53, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Li, J.Y.; Gosby, A.; To, S.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and its more biologically available derivative, Dihydroberberine, inhibit mitochondrial respiratory complex I. A mechanism for the action of berberine to activate AMP-Activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Kim, D.Y.; Chung, S.H. Caffeine attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. BMB Rep. 2013, 46, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Hsu, K.C.; Lee, J.W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Park, S.; Kim, M.J.; Yang, W.K.; UkIm, D.; Yang, K.R.; Hong, J.; Choe, W.; Kang, I.; Kim, S.S.; et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014, 281, 4421–4438. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Fresh Fruit | Dried Methanolic Extract |
|---|---|---|
| Total Polyphenols (mg GAeq/g) | 2.64 ± 2.63 | 23.44 ± 0.22 |
| Total Flavonoids (mg CATeq/g) | 1.02 ± 0.87 | 5.21 ± 0.29 |
| Vitamin C (mg/g) | 0.39 ± 0.23 | 9.09 ± 4.45 |
| Cyanidin-3-O-glucoside (mg/g) | 0.03 ± 0.02 | 0.69 ± 0.41 |
| Pelargonidin-3-O-glucoside (mg/g) | 0.70 ± 0.25 | 16.32 ± 5.71 |
| Pelargonidin-3-O-rutinoside (mg/g) | 0.04 ± 0.08 | 0.93 ± 1.39 |
| TAC (µmol Txeq/g) | ||
| FRAP | 22.70 ± 2.03 | 168.25 ± 3.95 |
| DPPH | 8.11 ± 0.25 | 30.29 ± 0.18 |
| TEAC | 10.71 ± 0.58 | 35.51 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Cordero, M.D.; Mezzetti, B.; Quiles, J.L.; Battino, M. Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation. Nutrients 2017, 9, 621. https://doi.org/10.3390/nu9060621
Forbes-Hernández TY, Giampieri F, Gasparrini M, Afrin S, Mazzoni L, Cordero MD, Mezzetti B, Quiles JL, Battino M. Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation. Nutrients. 2017; 9(6):621. https://doi.org/10.3390/nu9060621
Chicago/Turabian StyleForbes-Hernández, Tamara Y., Francesca Giampieri, Massimiliano Gasparrini, Sadia Afrin, Luca Mazzoni, Mario D. Cordero, Bruno Mezzetti, José L. Quiles, and Maurizio Battino. 2017. "Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation" Nutrients 9, no. 6: 621. https://doi.org/10.3390/nu9060621








