Long-Term Dexamethasone Exposure Down-Regulates Hepatic TFR1 and Reduces Liver Iron Concentration in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Data and Sample Collection
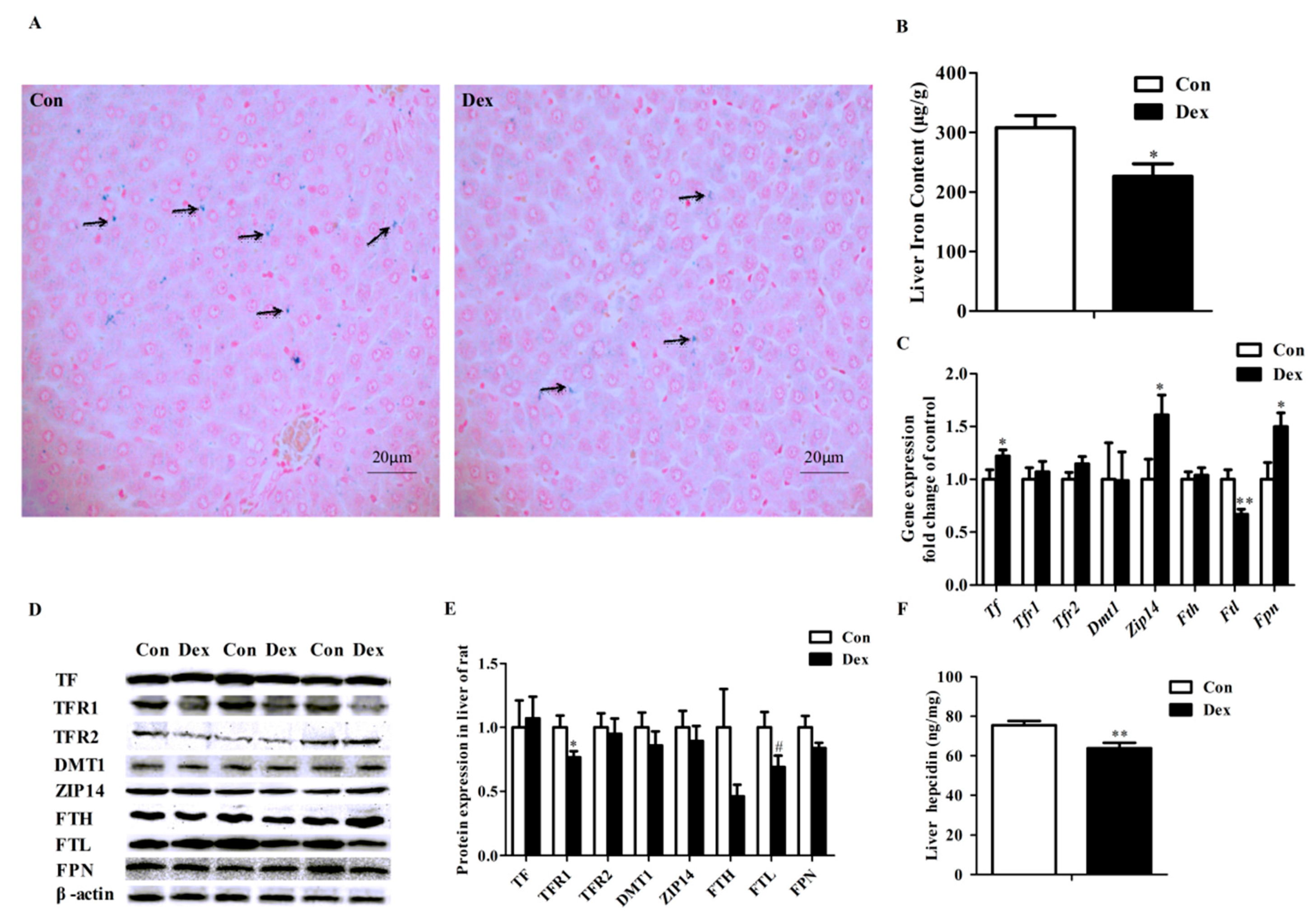
2.3. Histological Analysis of Liver
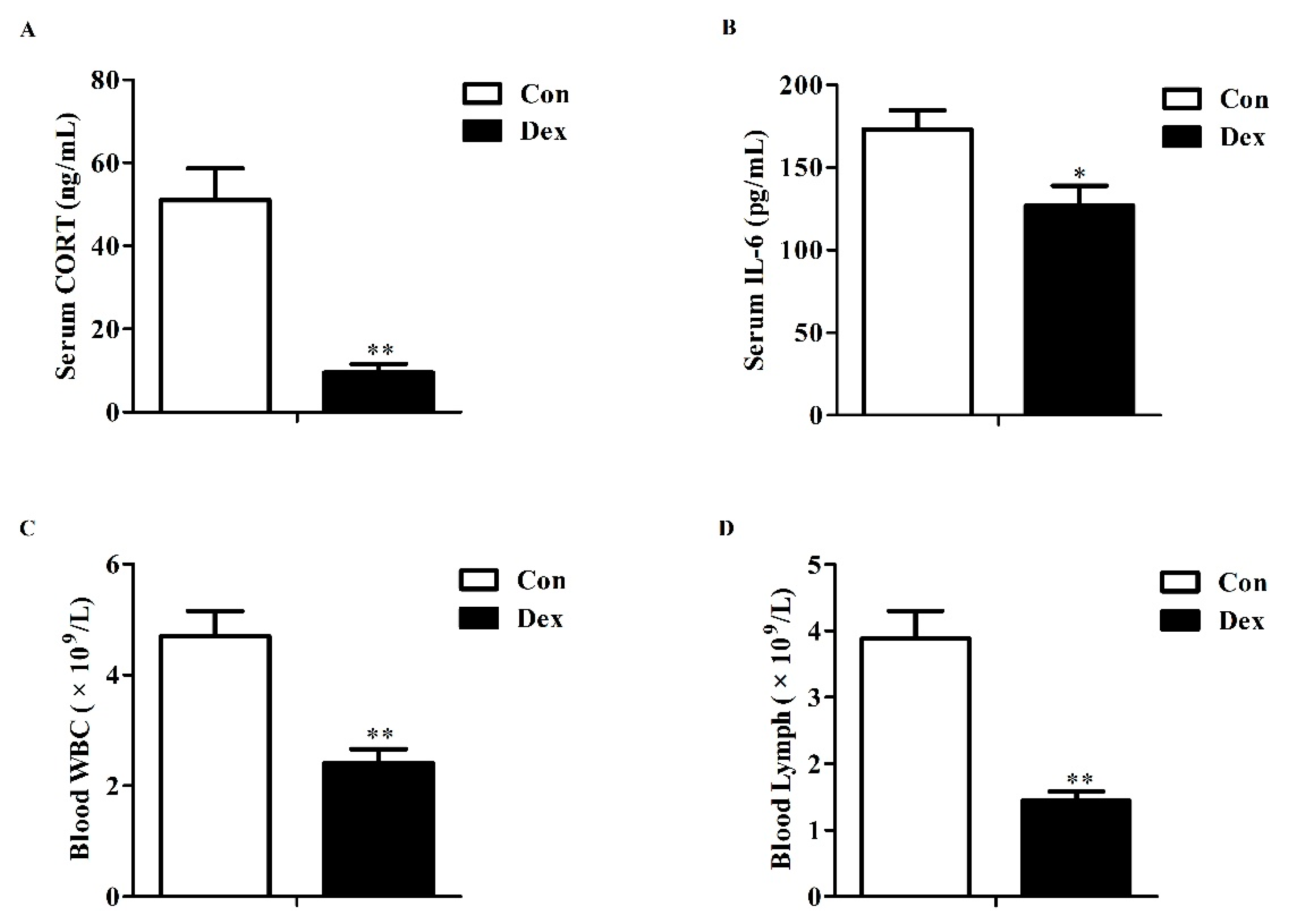
2.4. Haematological Parameters and Serum Concentration of Corticosterone, IL-6 and Iron Parameters
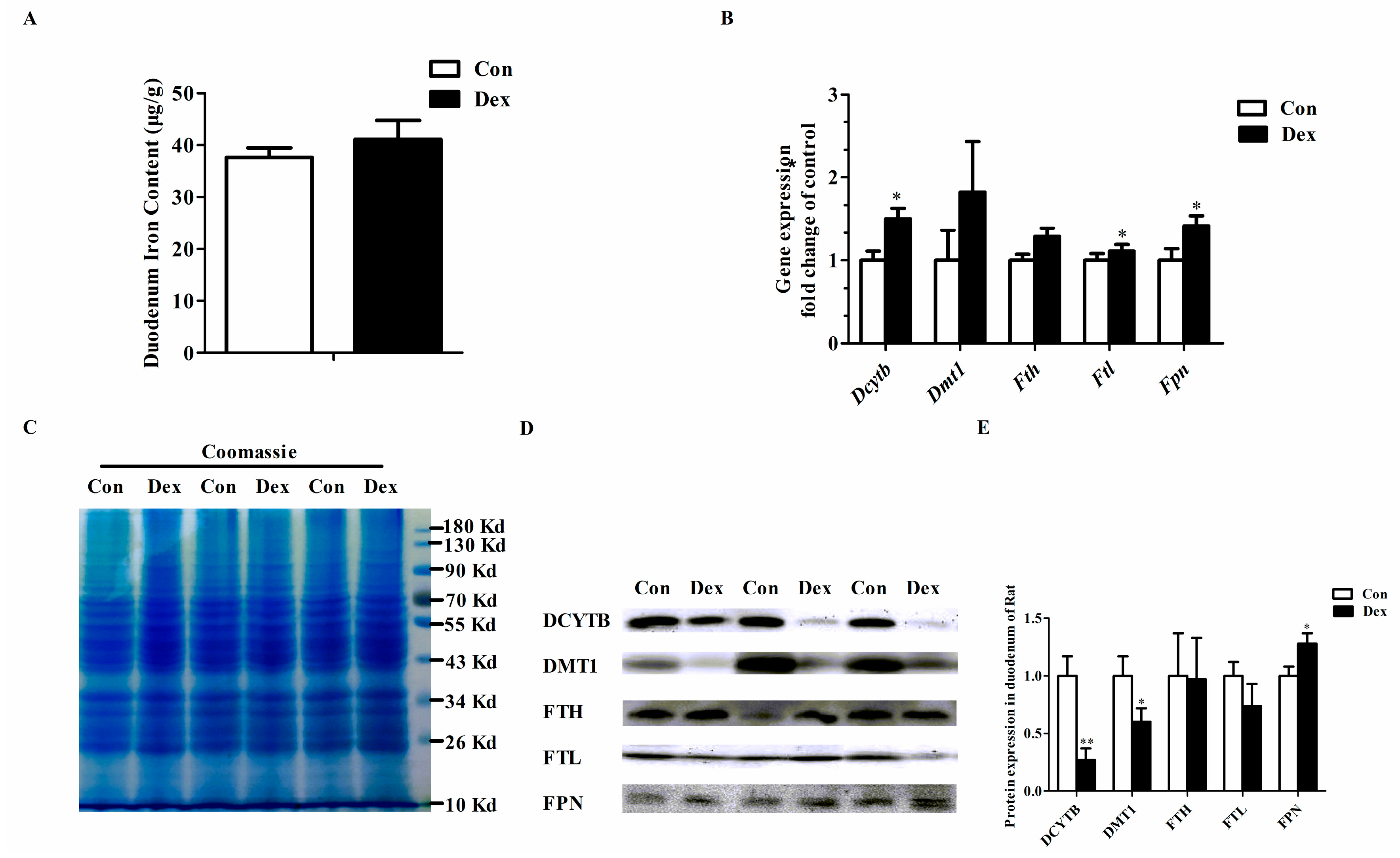
2.5. Iron Measurement in Tissues
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Total Protein Extractions and Western Blotting Analysis
2.8. RNA Electrophoretic Mobility Shift Assay (REMSA)
2.9. Statistical Analysis
3. Results
3.1. Serum Corticosterone, IL-6 and Blood Parameters
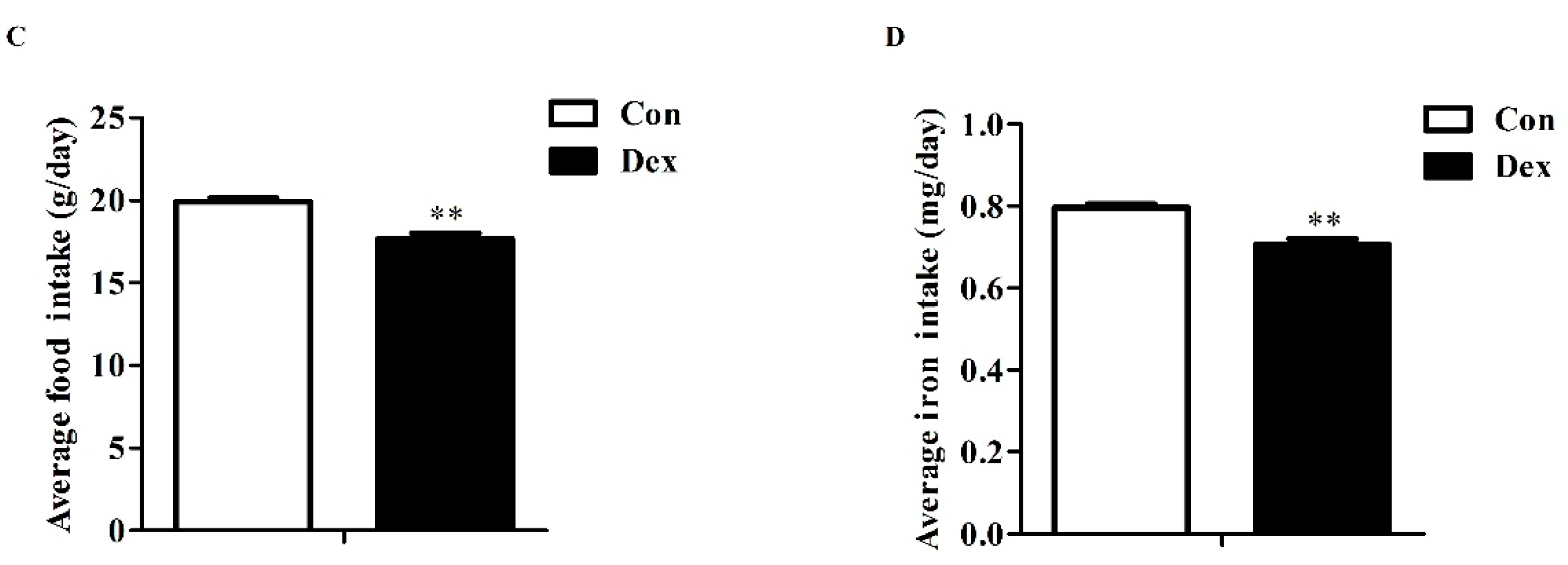
3.2. Body Weight, Food Intake and Iron Intake
3.3. Serum Iron Parameters
3.4. Duodenal Expression of Iron Metabolism-Related Genes
3.5. Histological Characteristics, Hepatic Iron Content and Hepatic Iron-Metabolism Genes Expression
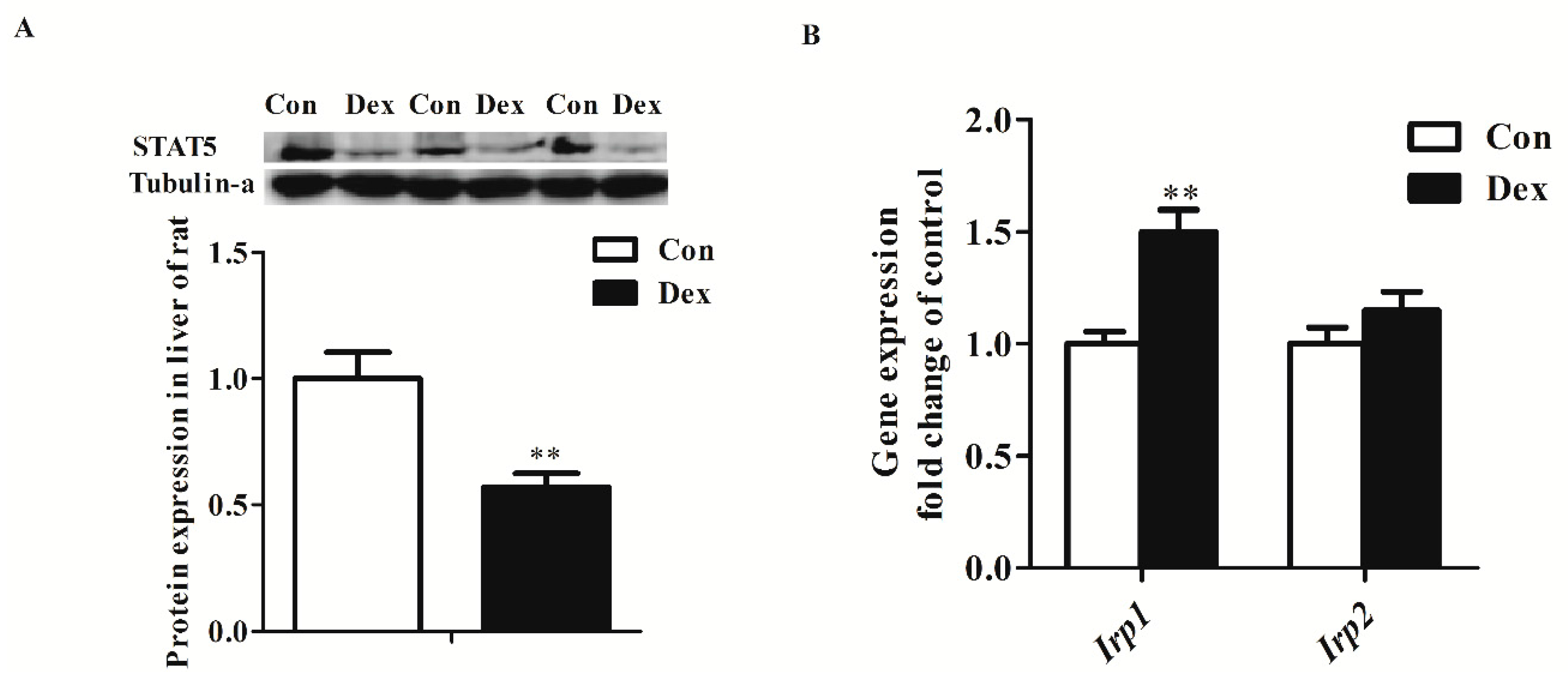
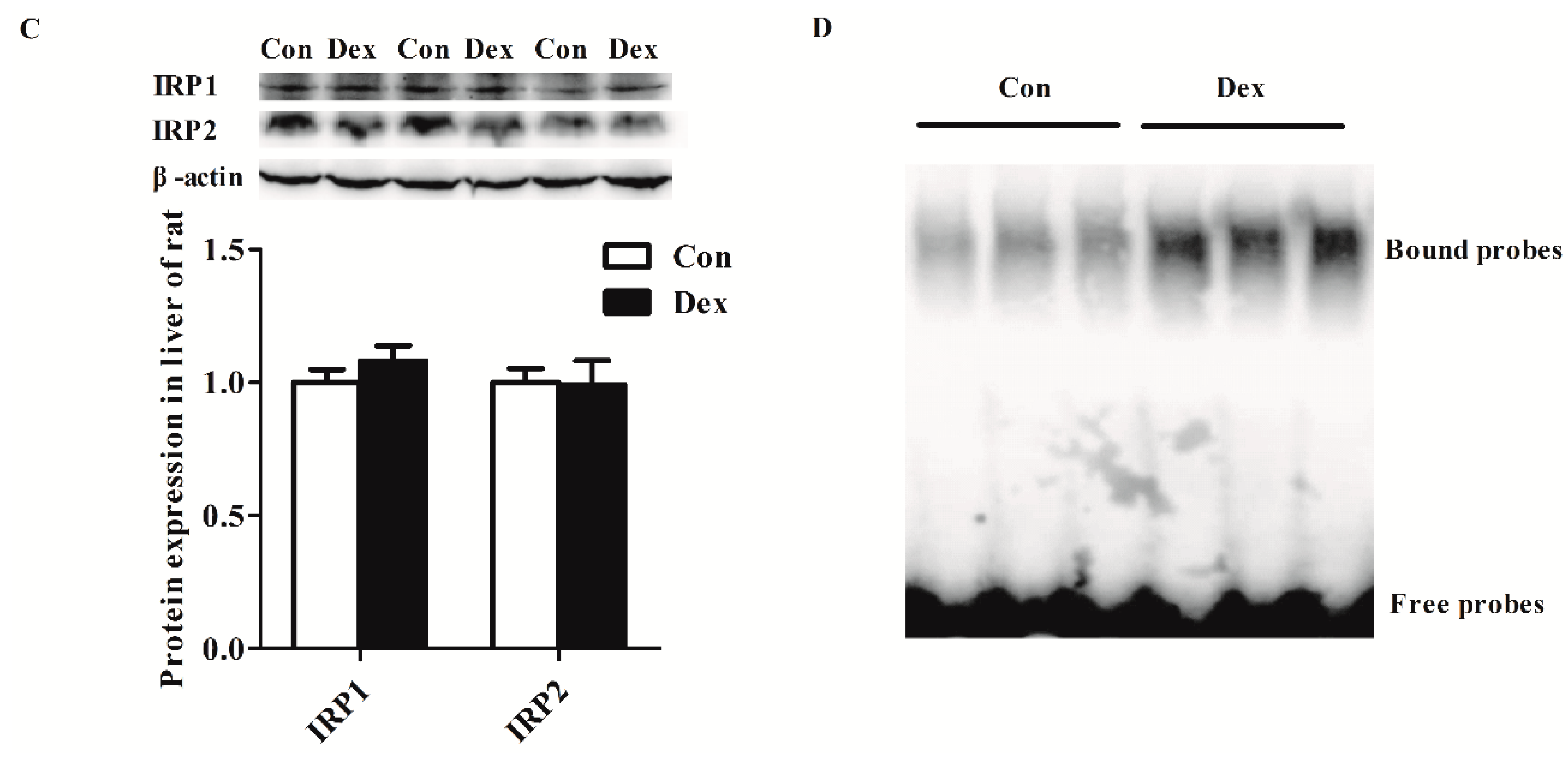
3.6. Transcription and Post-Transcriptional Regulation of Hepatic Tfr1
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chifman, J.; Laubenbacher, R.; Torti, S.V. A systems biology approach to iron metabolism. Adv. Exp. Med. Biol. 2014, 844, 201–225. [Google Scholar] [PubMed]
- Andrews, N.C. Forging a field: The golden age of iron biology. Blood 2008, 112, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Tandara, L.; Salamunic, I. Iron metabolism: Current facts and future directions. Biochem. Med. 2012, 22, 311–328. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta 2012, 9, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.P.; Shen, M.; Eisenstein, R.S.; Leibold, E.A. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. Biophys. Acta 2012, 1823, 1468–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.L.; Bilbo, S.D.; Dhabhar, F.S.; Nelson, R.J. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav. Immun. 2008, 22, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.F.; Sun, W.M.; Shi, L.F.; Hou, D.D.; Liu, H. Effects of restraint stress on iron, zinc, calcium, and magnesium whole blood levels in mice. Biol. Trace Elem. Res. 2008, 121, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhou, J.; Huang, X.; Li, M. Effects of psychological stress on serum iron and erythropoiesis. Int. J. Hematol. 2008, 88, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.; Wang, W.; Wang, L.; Ma, L.; Shen, H.; Li, M. Psychological stress induces hypoferremia through the il-6-hepcidin axis in rats. Biochem. Biophys. Res. Commun. 2008, 373, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, H.; Chen, C.; Wang, W.; Yu, S.; Zhao, M.; Li, M. The effect of psychological stress on iron absorption in rats. BMC Gastroenterol. 2009, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ma, L.; Wang, H.; Shen, Z.; Li, M. Glucocorticoid causes iron accumulation in liver by up-regulating expression of iron regulatory protein 1 gene through gr and stat5. Cell Biochem. Biophys. 2011, 61, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J.; Summers, A.P. Tendon—Bridging the gap. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 905–1192. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Farajdokht, F.; Soleimani, M.; Mehrpouya, S.; Barati, M.; Nahavandi, A. The role of hepcidin in chronic mild stress-induced depression. Neurosci. Lett. 2015, 588, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Sapolsky, R.M. An inflammatory review of glucocorticoid actions in the cns. Brain Behav Immun. 2007, 21, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Teague, C.R.; Dhabhar, F.S.; Barton, R.H.; Beckwith-Hall, B.; Powell, J.; Cobain, M.; Singer, B.; McEwen, B.S.; Lindon, J.C.; Nicholson, J.K.; et al. Metabonomic studies on the physiological effects of acute and chronic psychological stress in sprague-dawley rats. J. Proteome Res. 2007, 6, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, A.; Priftis, K.N. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Jahng, J.W.; Kim, N.Y.; Ryu, V.; Yoo, S.B.; Kim, B.T.; Kang, D.W.; Lee, J.H. Dexamethasone reduces food intake, weight gain and the hypothalamic 5-ht concentration and increases plasma leptin in rats. Eur. J. Pharmacol. 2008, 581, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, H.Y.; Liu, G.H.; Dong, X.L.; Chang, W.H.; Zhang, S.; Zheng, A.J.; Chen, G.L. Effects of stress simulated by dexamethasone on jejunal glucose transport in broilers. Poult. Sci. 2009, 88, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Thachil, A.J.; Shaw, D.P.; Nagaraja, K.V. Effects of dexamethasone immunosuppression on turkey clostridial dermatitis. Avian Dis. 2014, 58, 433–436. [Google Scholar] [CrossRef] [PubMed]
- LaVaute, T.; Smith, S.; Cooperman, S.; Iwai, K.; Land, W.; Meyron-Holtz, E.; Drake, S.K.; Miller, G.; Abu-Asab, M.; Tsokos, M.; et al. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat. Genet. 2001, 27, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gu, Y.; Lu, J.; Yuan, L.; Zhao, R. Effects of chromium propionate on egg production, egg quality, plasma biochemical parameters, and egg chromium deposition in late-phase laying hens. Biol. Trace Elem. Res. 2014, 157, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Marti, O.; Marti, J.; Armario, A. Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure. Physiol. Behav. 1994, 55, 747–753. [Google Scholar] [CrossRef]
- Cole, M.A.; Kim, P.J.; Kalman, B.A.; Spencer, R.L. Dexamethasone suppression of corticosteroid secretion: Evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology 2000, 25, 151–167. [Google Scholar] [CrossRef]
- Simoens, V.L.; Hebert, S. Cortisol suppression and hearing thresholds in tinnitus after low-dose dexamethasone challenge. BMC Ear Nose Throat Disord. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Rybkin, I.I.; Zhou, Y.; Volaufova, J.; Smagin, G.N.; Ryan, D.H.; Harris, R.B. Effect of restraint stress on food intake and body weight is determined by time of day. Am. J. Physiol. 1997, 273, R1612–R1622. [Google Scholar]
- Valles, A.; Marti, O.; Garcia, A.; Armario, A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1138–R1144. [Google Scholar] [PubMed]
- Caldefie-Chezet, F.; Poulin, A.; Enreille-Leger, A.; Vasson, M.P. Troglitazone reduces leptinemia during experimental dexamethasone-induced stress. Horm. Metab. Res. 2005, 37, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zheng, Y.; Shen, Z.; Li, Y.; Tian, X.; Dou, X.; Qian, J.; Shen, H. Psychological stress-induced lower serum zinc and zinc redistribution in rats. Biol. Trace Elem. Res. 2013, 155, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zheng, Y.; Li, Y.; Shen, Z.; Tao, L.; Dou, X.; Qian, J.; Shen, H. Psychological stress induced zinc accumulation and up-regulation of zip14 and metallothionein in rat liver. BMC Gastroenterol. 2014, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Wang, Y.; Ricci, M.R.; Sullivan, S.; Russell, C.D.; Fried, S.K. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E858–E864. [Google Scholar] [CrossRef] [PubMed]
- Cartmill, J.A.; Thompson, D.L., Jr.; Storer, W.A.; Crowley, J.C.; Huff, N.K.; Waller, C.A. Effect of dexamethasone, feeding time, and insulin infusion on leptin concentrations in stallions. J. Anim. Sci. 2005, 83, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Vignjevic, S.; Budec, M.; Markovic, D.; Dikic, D.; Mitrovic, O.; Mojsilovic, S.; Duric, S.V.; Koko, V.; Cokic, B.B.; Cokic, V.; et al. Chronic psychological stress activates bmp4-dependent extramedullary erythropoiesis. J. Cell. Mol. Med. 2014, 18, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Latunde-Dada, G.O.; Simpson, R.J.; McKie, A.T. Duodenal cytochrome b expression stimulates iron uptake by human intestinal epithelial cells. J. Nutr. 2008, 138, 991–995. [Google Scholar] [PubMed]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; Direnzo, C.; Robine, S.; Andrews, N.C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Investig. 2005, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Ohrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal dmt1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Sugianto, P.; Fung, E.; Del-Castillo-Rueda, A.; Moran-Jimenez, M.J.; Ganz, T.; Nemeth, E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012, 15, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through stat3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; McHugh, K.; Drakesmith, H. Regulation of hepcidin by erythropoiesis: The story so far. Annu. Rev. Nutr. 2016, 36, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.M.; Chua, A.C.; Herbison, C.E.; Olynyk, J.K.; Trinder, D. Liver iron transport. World J. Gastroenterol. 2007, 13, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar] [PubMed]
- Graham, R.M.; Reutens, G.M.; Herbison, C.E.; Delima, R.D.; Chua, A.C.; Olynyk, J.K.; Trinder, D. Transferrin receptor 2 mediates uptake of transferrin-bound and non-transferrin-bound iron. J. Hepatol. 2008, 48, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Kerenyi, M.A.; Grebien, F.; Gehart, H.; Schifrer, M.; Artaker, M.; Kovacic, B.; Beug, H.; Moriggl, R.; Mullner, E.W. Stat5 regulates cellular iron uptake of erythroid cells via irp-2 and tfr-1. Blood 2008, 112, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Miell, J.P.; Corder, R.; Pralong, F.P.; Gaillard, R.C. Effects of dexamethasone on growth hormone (gh)-releasing hormone, arginine- and dopaminergic stimulated gh secretion, and total plasma insulin-like growth factor-i concentrations in normal male volunteers. J. Clin. Endocrinol. Metab. 1991, 72, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Jux, C.; Leiber, K.; Hugel, U.; Blum, W.; Ohlsson, C.; Klaus, G.; Mehls, O. Dexamethasone impairs growth hormone (gh)-stimulated growth by suppression of local insulin-like growth factor (igf)-i production and expression of gh- and igf-i-receptor in cultured rat chondrocytes. Endocrinology 1998, 139, 3296–3305. [Google Scholar] [CrossRef] [PubMed]
- Bonnah, R.A.; Muckenthaler, M.U.; Carlson, H.; Minana, B.; Enns, C.A.; Hentze, M.W.; So, M. Expression of epithelial cell iron-related genes upon infection by neisseria meningitidis. Cell. Microbiol. 2004, 6, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.; Horowitz, J.A.; Basilion, J.P.; Koeller, D.M.; Klausner, R.D.; Harford, J.B. Evidence that the pathway of transferrin receptor mrna degradation involves an endonucleolytic cleavage within the 3′ utr and does not involve poly(a) tail shortening. EMBO J. 1994, 13, 1969–1980. [Google Scholar] [PubMed]
- Hopgood, M.F.; Clark, M.G.; Ballard, F.J. Stimulation by glucocorticoids of protein degradation in hepatocyte monolayers. Biochem. J. 1981, 196, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Wang, W.; Zhang, C.; Liu, C.; Lu, J.; Li, W.; Zuo, R.; Myatt, L.; Sun, K. Autophagic degradation of collagen 1a1 by cortisol in human amnion fibroblasts. Endocrinology 2017, 158, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Trausch-Azar, J.S.; Muglia, L.J.; Schwartz, A.L. Glucocorticoids differentially regulate degradation of myod and id1 by n-terminal ubiquitination to promote muscle protein catabolism. PNAS 2008, 105, 3339–3344. [Google Scholar] [CrossRef] [PubMed]
- Lilienbaum, A. Relationship between the proteasomal system and autophagy. Int. J. Biochem. Mol. Biol. 2013, 4, 1–26. [Google Scholar] [PubMed]

| Target Genes | Sequences (5′ to 3′) | Products | GenBank No. | |
|---|---|---|---|---|
| Dcytb | F:agacttggacgaggatact | R:ggcagaccaggatatgtg | 104 bp | NM_001011954.1 |
| Dmt1 | F:tcacttggtcctcgttct | R:tcactaacagcctccttatag | 139 bp | NM_013173.2 |
| Fth | F:gtcactactggaacttcaca | R:ttcaggtaatgcgtctcaat | 216 bp | NM_012848.2 |
| Ftl | F:gcagaagccatctcaaga | R:ttccaagaagtcacagagg | 197 bp | NM_022500.4 |
| Fpn | F:aggaaggatgctgtggat | R:tgtcaagaggaggctgtt | 115 bp | NM_133315.2 |
| Tf | F:atcagactccagcatcaac | R:ccaatacacaggtcacaga | 198 bp | NM_001013110.1 |
| Tfr1 | F:cacttacggtcagcactt | R:cacaactcactggacttaga | 114 bp | NM_022712.1 |
| Tfr2 | F:gttggtggttggtgaaga | R:acatagtgcgtgtcagtc | 240 bp | NM_001105916.1 |
| Zip14 | F:ttggaagaagcactgagag | R:ttggaagaagcactgagag | 149 bp | NM_001107275.1 |
| Irp1 | F:cgatgctgtgaagaagttg | R:aatgaacctggatggaatga | 92 bp | NM_017321.1 |
| Irp2 | F:ggcacagattctcatataacc | R:tcacatccaaccacctct | 131 bp | NM_022863.2 |
| Arbp | F:tagagggtgtccgcaatgtg | R:cagtgggaaggtgtagtcagtc | 217 bp | NM_022402.2 |
| Parameters | Con | Dex | P-Value |
|---|---|---|---|
| Serum iron (µmol/L) | 45.29 ± 2.12 | 55.09 ± 2.80 | 0.01 |
| UIBC (µmol/L) | 37.19 ± 2.02 | 37.24 ± 4.10 | 0.20 |
| TIBC (µmol/L) | 82.48 ± 2.06 | 92.33 ± 3.44 | 0.20 |
| Transferrin (µg/dL) | 131.40 ± 2.64 | 135.20 ± 3.21 | 0.37 |
| TS (%) | 52.59 ± 2.82 | 60.28 ± 3.53 | 0.11 |
| Ferritin (ng/mL) | 6.85 ± 0.34 | 7.34 ± 0.80 | 0.60 |
| sTfR (nmol/L) | 32.29 ± 8.10 | 135.42 ± 38.96 | 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Jiang, S.; Yang, C.; Yang, S.; He, B.; Ma, W.; Zhao, R. Long-Term Dexamethasone Exposure Down-Regulates Hepatic TFR1 and Reduces Liver Iron Concentration in Rats. Nutrients 2017, 9, 617. https://doi.org/10.3390/nu9060617
Li H, Jiang S, Yang C, Yang S, He B, Ma W, Zhao R. Long-Term Dexamethasone Exposure Down-Regulates Hepatic TFR1 and Reduces Liver Iron Concentration in Rats. Nutrients. 2017; 9(6):617. https://doi.org/10.3390/nu9060617
Chicago/Turabian StyleLi, Huifang, Shuxia Jiang, Chun Yang, Shu Yang, Bin He, Wenqiang Ma, and Ruqian Zhao. 2017. "Long-Term Dexamethasone Exposure Down-Regulates Hepatic TFR1 and Reduces Liver Iron Concentration in Rats" Nutrients 9, no. 6: 617. https://doi.org/10.3390/nu9060617




