A High-Protein Diet Reduces Weight Gain, Decreases Food Intake, Decreases Liver Fat Deposition, and Improves Markers of Muscle Metabolism in Obese Zucker Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plasma Analysis
2.3. Muscle and Liver Histology
2.4. RNA Isolation and Real-Time PCR
2.5. Western Blot Analysis
2.6. Statistics
3. Results
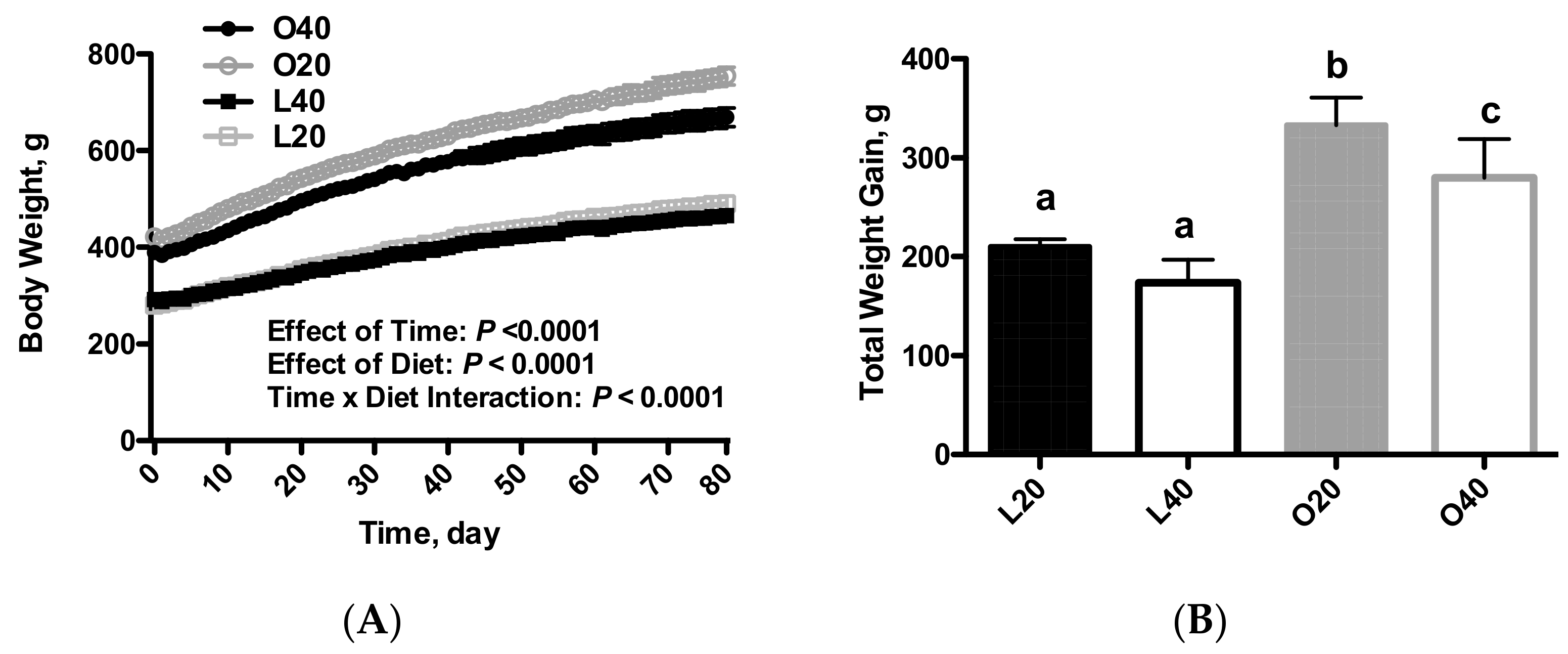
3.1. Body Weight, Food Intake and Body Composition (Tissue Weights)
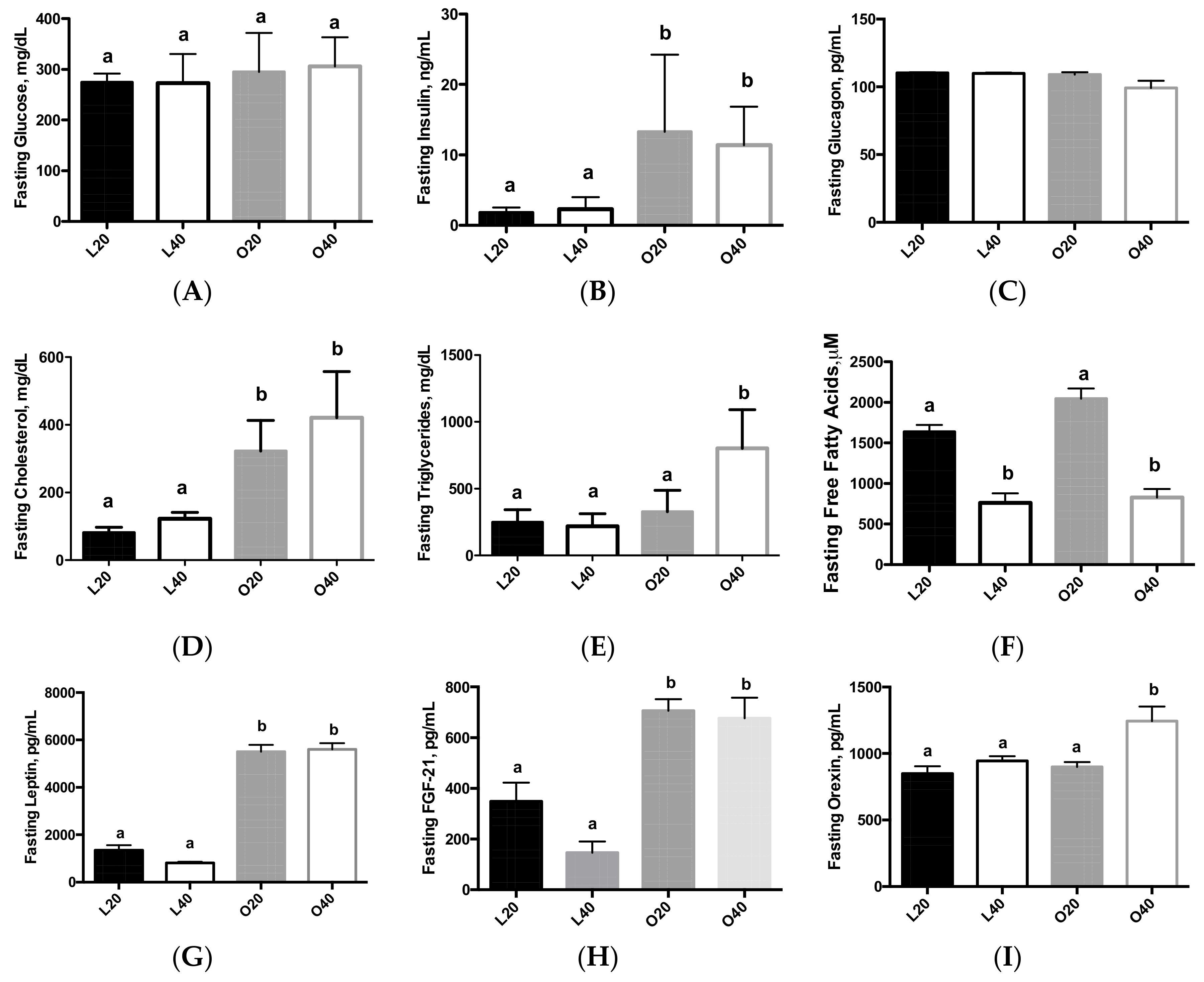
3.2. Biomarkers of Obesity and Diabetes
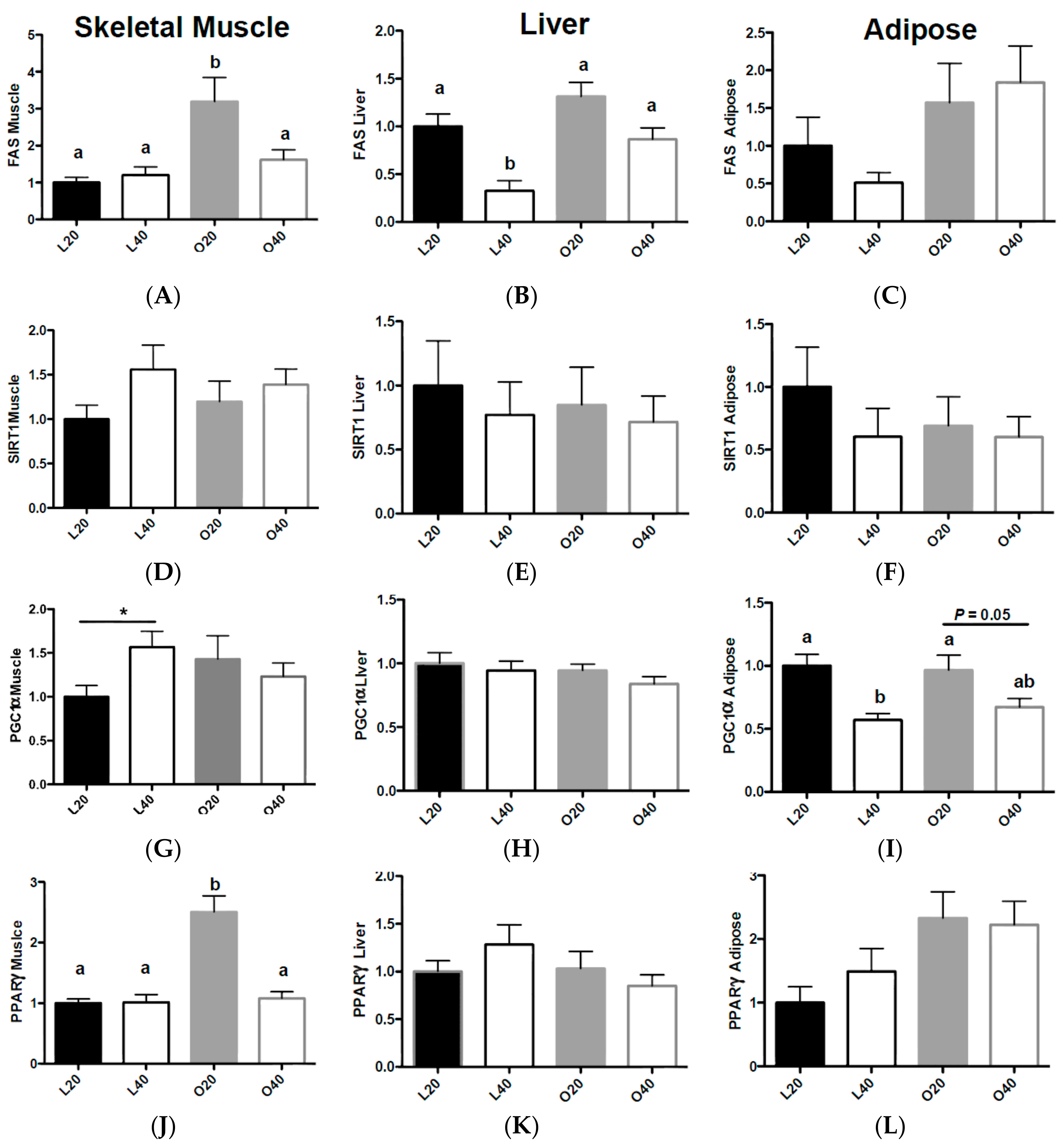
3.3. Gene Expression Related to Fat Deposition and Energy Metabolism
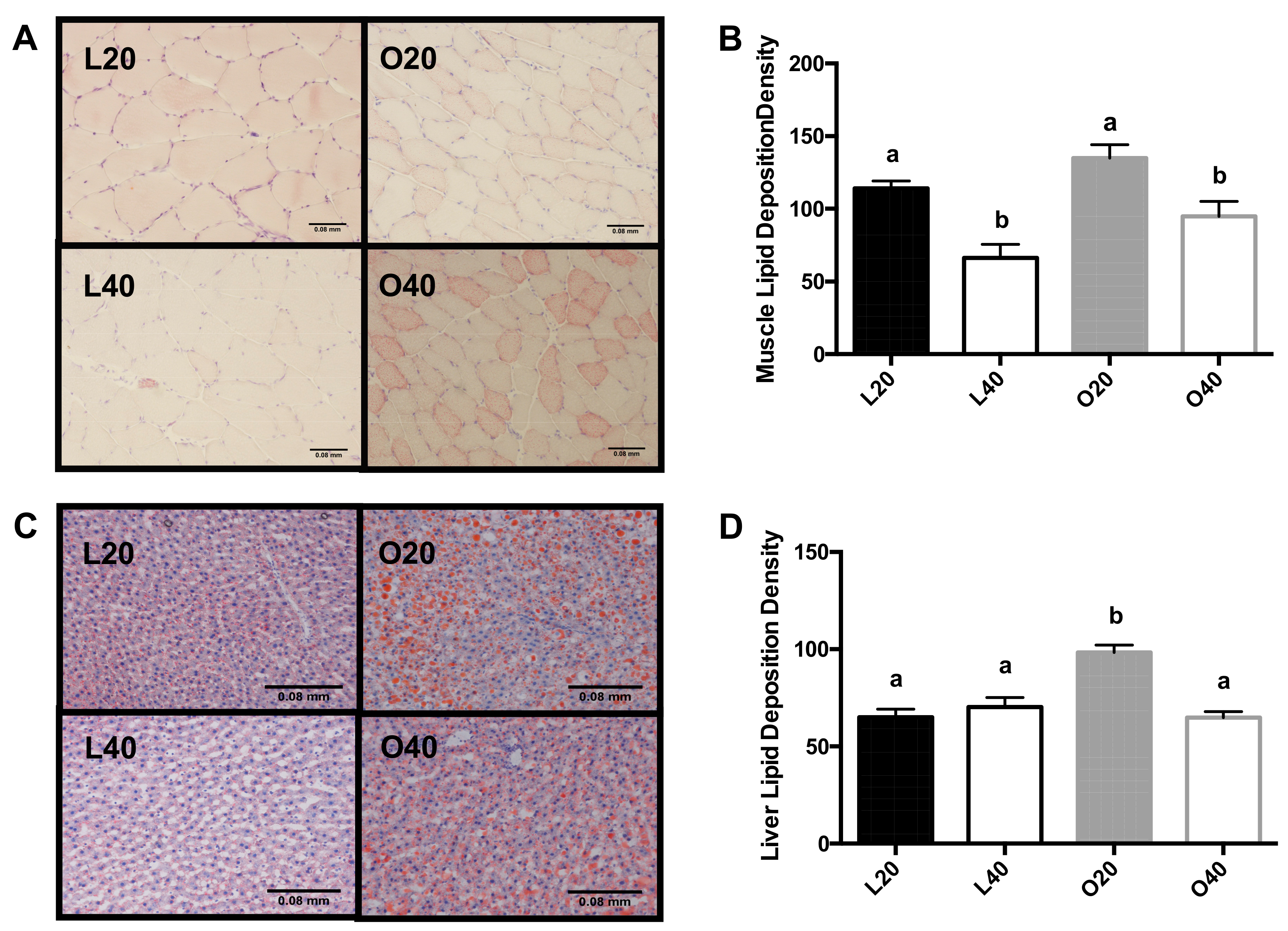
3.4. Tissue Neutral Lipid Deposition
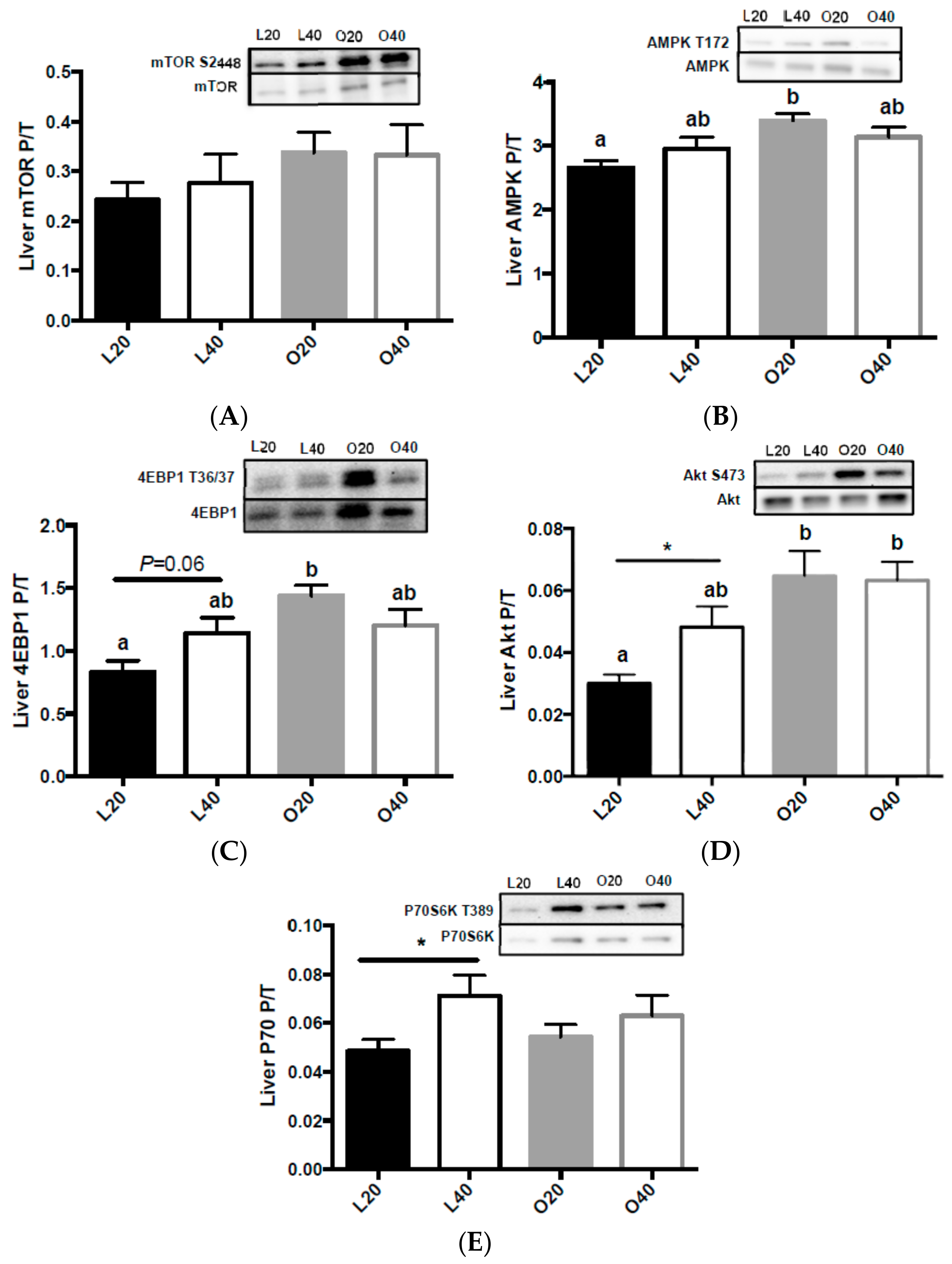
3.5. Metabolic Signaling
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4E-BP1 | eukaryotic initiation 4E factor binding protein 1 |
| ADMR | acceptable macronutrient distribution range |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| FAS | fatty acid synthase |
| FGF-21 | fibroblast growth factor-21 |
| IGF-1 | insulin-like growth factor-1 |
| L20 | lean animals on 20% protein diet |
| L40 | lean animals on 40% protein diet |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| O20 | obese animals on 20% protein diet |
| O40 | obese animals on 40% protein diet |
| p70S6K | ribosomal protein S6 kinase |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| SIRT1 | sirtuin 1 |
References
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will all americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008, 16, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Insulin resistance: The link between obesity and cardiovascular disease. Med. Clin. N. Am. 2011, 95, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Board, F.A.N. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; Medicine, I.O., Ed.; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Farnsworth, E.; Luscombe, N.D.; Noakes, M.; Wittert, G.; Argyiou, E.; Clifton, P.M. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am. J. Clin. Nutr. 2003, 78, 31–39. [Google Scholar] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 734–741. [Google Scholar] [PubMed]
- Layman, D.K.; Shiue, H.; Sather, C.; Erickson, D.J.; Baum, J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J. Nutr. 2003, 133, 405–410. [Google Scholar] [PubMed]
- Layman, D.K.; Boileau, R.A.; Erickson, D.J.; Painter, J.E.; Shiue, H.; Sather, C.; Christou, D.D. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J. Nutr. 2003, 133, 411–417. [Google Scholar] [PubMed]
- Baum, J.I.; Gray, M.; Binns, A. Breakfasts higher in protein increase postprandial energy expenditure, increase fat oxidation, and reduce hunger in overweight children from 8 to 12 years of age. J. Nutr. 2015, 145, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Layman, D.K.; Freund, G.G.; Rahn, K.A.; Nakamura, M.T.; Yudell, B.E. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J. Nutr. 2006, 136, 1855–1861. [Google Scholar] [PubMed]
- Devkota, S.; Layman, D.K. Increased ratio of dietary carbohydrate to protein shifts the focus of metabolic signaling from skeletal muscle to adipose. Nutr. Metab. (Lond.) 2011, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Tinker, L.F.; Sarto, G.E.; Howard, B.V.; Huang, Y.; Neuhouser, M.L.; Mossavar-Rahmani, Y.; Beasley, J.M.; Margolis, K.L.; Eaton, C.B.; Phillips, L.S.; et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the women’s health initiative. Am. J. Clin. Nutr. 2011, 94, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; van der A, D.L.; Spijkerman, A.M.; Grobbee, D.E.; van der Schouw, Y.T. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the european prospective investigation into cancer and nutrition (epic)-nl study. Diabetes Care 2010, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Van Nielen, M.; Feskens, E.J.; Mensink, M.; Sluijs, I.; Molina, E.; Amiano, P.; Ardanaz, E.; Balkau, B.; Beulens, J.W.; Boeing, H.; et al. Dietary protein intake and incidence of type 2 diabetes in europe: The epic-interact case-cohort study. Diabetes Care 2014, 37, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Halkjaer, J.; Olsen, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; Buijsse, B.; Palli, D.; Tognon, G.; Du, H.; van der A, D.L.; et al. Intake of total, animal and plant protein and subsequent changes in weight or waist circumference in european men and women: The diogenes project. Int. J. Obes. (Lond.) 2011, 35, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [PubMed]
- Heger, J. Essential to non-essential amino acid ratios. In Amino Acids in Animal Nutrition, 2nd ed.; D’Mello, J.D.F., Ed.; CAB International: Wallingford, UK, 2003; pp. 103–124. [Google Scholar]
- Simoni, R.D.; Hill, R.L.; Vaughan, M. The amino-acid minimum for maintenance and growth, as exemplified by further experiments with lysine and tryptophane (Osborne, T.B.; Mendel, L.B. J. Biol. Chem. 1916, 25, 1–12) and the role of vitamines in the diet (Osborne, T.B.; Mendel, L.B. J. Biol. Chem. 1917, 31, 149–163). J. Biol. Chem. 2002, 277, E7. [Google Scholar]
- Layman, D.K.; Baum, J.I. Dietary protein impact on glycemic control during weight loss. J. Nutr. 2004, 134, 968S–973S. [Google Scholar] [PubMed]
- Van Loon, L.J. Leucine as a pharmaconutrient in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermudez-Silva, F.J.; Elie, M.; Leste-Lasserre, T.; Belluomo, I.; Clark, S.; Duchampt, A.; Mithieux, G.; Cota, D. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity (Silver Spring) 2014, 22, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J. Nutr. Biochem. 2012, 23, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Freudenberg, A.; Klaus, S. Beyond the role of dietary protein and amino acids in the prevention of diet-induced obesity. Int. J. Mol. Sci. 2014, 15, 1374–1391. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; O’Connor, J.C.; Seyler, J.E.; Anthony, T.G.; Freund, G.G.; Layman, D.K. Leucine reduces the duration of insulin-induced pi 3-kinase activity in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E86–E91. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.D.; Baum, J.I.; Kimball, S.R.; Jefferson, L.S. Mechanisms involved in the coordinate regulation of mtorc1 by insulin and amino acids. J. Biol. Chem. 2011, 286, 8287–8296. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Washington, T.A.; Shouse, S.A.; Bottje, W.; Dridi, S.; Davis, G.; Smith, D. Leucine supplementation at the onset of high-fat feeding does not prevent weight gain or improve glycemic regulation in male sprague-dawley rats. J. Physiol. Biochem. 2016, 72, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.J.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C.; Blom, W.A.; Smeets, P.A.; Stafleu, A.; Hendriks, H.F. Biomarkers of satiation and satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [PubMed]
- Patti, M.E.; Brambilla, E.; Luzi, L.; Landaker, E.J.; Kahn, C.R. Bidirectional modulation of insulin action by amino acids. J. Clin. Investig. 1998, 101, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mtor/p70 s6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [PubMed]
- Gautsch, T.A.; Anthony, J.C.; Kimball, S.R.; Paul, G.L.; Layman, D.K.; Jefferson, L.S. Availability of eif4e regulates skeletal muscle protein synthesis during recovery from exercise. Am. J. Physiol. 1998, 274, C406–C414. [Google Scholar] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [PubMed]
- Festuccia, W.T.; Blanchard, P.G.; Belchior, T.; Chimin, P.; Paschoal, V.A.; Magdalon, J.; Hirabara, S.M.; Simoes, D.; St-Pierre, P.; Carpinelli, A.; et al. Ppargamma activation attenuates glucose intolerance induced by mtor inhibition with rapamycin in rats. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1046–E1054. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. Mtor: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. Mtor signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Chen, J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 2004, 53, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.G.; Festuccia, W.T.; Houde, V.P.; St-Pierre, P.; Brule, S.; Turcotte, V.; Cote, M.; Bellmann, K.; Marette, A.; Deshaies, Y. Major involvement of mtor in the ppargamma-induced stimulation of adipose tissue lipid uptake and fat accretion. J. Lipid Res. 2012, 53, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kuchipudi, S.V.; Tellabati, M.; Nelli, R.K.; White, G.A.; Perez, B.B.; Sebastian, S.; Slomka, M.J.; Brookes, S.M.; Brown, I.H.; Dunham, S.P.; et al. 18s rrna is a reliable normalisation gene for real time pcr based on influenza virus infected cells. Virol. J. 2012, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, K.; Greene, E.; Piekarski, A.; Faulkner, O.B.; Hargis, B.M.; Bottje, W.; Dridi, S. Orexin system is expressed in avian muscle cells and regulates mitochondrial dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R173–R187. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Kimball, S.R.; Jefferson, L.S. Glucagon acts in a dominant manner to repress insulin-induced mammalian target of rapamycin complex 1 signaling in perfused rat liver. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E410–E415. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Lieberman, H.R.; Fulgoni, V.L., 3rd. Higher-protein diets are associated with higher HDL cholesterol and lower bmi and waist circumference in us adults. J. Nutr. 2015, 145, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. (Lond.) 2014, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kinsey-Jones, J.S.; Alamshah, A.; McGavigan, A.K.; Spreckley, E.; Banks, K.; Cereceda Monteoliva, N.; Norton, M.; Bewick, G.A.; Murphy, K.G. Gprc6a is not required for the effects of a high-protein diet on body weight in mice. Obesity (Silver Spring) 2015, 23, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- McGavigan, A.K.; O’Hara, H.C.; Amin, A.; Kinsey-Jones, J.; Spreckley, E.; Alamshah, A.; Agahi, A.; Banks, K.; France, R.; Hyberg, G.; et al. L-cysteine suppresses ghrelin and reduces appetite in rodents and humans. Int. J. Obes. (Lond.) 2015, 39, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, J.D.; Webster, A.J. The effect of varying the quality of dietary protein and energy on food intake and growth in the zucker rat. Br. J. Nutr. 1979, 41, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Silvestre, M.P.; Poppitt, S.D. Prevention of type 2 diabetes through lifestyle modification: Is there a role for higher-protein diets? Adv. Nutr. 2015, 6, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mtor signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.; Zampieri, T.T.; Donato, J., Jr. Reviewing the effects of l-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients 2015, 7, 3914–3937. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, D.O.; Knight, D.S.; Hamilton, K.; Tulp, O.; Tso, P. Localization of leptin receptor immunoreactivity in the lean and obese zucker rat brain. Brain Res. 1998, 785, 80–90. [Google Scholar] [CrossRef]
- Dunn, M.A.; Hartsook, E.W. Comparative amino acid and protein metabolism in obese and non-obese zucker rats. J. Nutr. 1980, 110, 1865–1879. [Google Scholar] [PubMed]
- Stengel, A.; Goebel-Stengel, M.; Wang, L.; Hu, E.; Karasawa, H.; Pisegna, J.R.; Tache, Y. High-protein diet selectively reduces fat mass and improves glucose tolerance in western-type diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R582–R591. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Rachek, L.I. Free fatty acids and skeletal muscle insulin resistance. Prog. Mol. Biol. Transl. Sci. 2014, 121, 267–292. [Google Scholar] [PubMed]
- Clifton, P.M.; Keogh, J.B.; Noakes, M. Long-term effects of a high-protein weight-loss diet. Am. J. Clin. Nutr. 2008, 87, 23–29. [Google Scholar] [PubMed]
- Pedersen, E.; Jesudason, D.R.; Clifton, P.M. High protein weight loss diets in obese subjects with type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, J.L.; Devassy, J.G.; Wu, Y.; Zahradka, P.; Taylor, C.G.; Aukema, H.M. Protein source in a high-protein diet modulates reductions in insulin resistance and hepatic steatosis in fa/fa zucker rats. Obesity (Silver Spring) 2016, 24, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.R.; Liao, Y.H.; Ding, Z.; Hara, D.; Kleinert, M.; Nelson, J.L.; Ivy, J.L. An amino acid mixture improves glucose tolerance and lowers insulin resistance in the obese zucker rat. Amino Acids 2013, 45, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, X.; Zheng, D.; Jiang, X.; Chen, Q. Branched-chain amino acids supplementation protects streptozotocin-induced insulin secretion and the correlated mechanism. Biofactors 2015, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Olson, K.C.; Kadota, Y.; Inukai, A.; Shimomura, Y.; Hoppel, C.L.; Adams, S.H.; Kawamata, Y.; Matsumoto, H.; Sakai, R.; et al. Leucine and protein metabolism in obese zucker rats. PLoS ONE 2013, 8, e59443. [Google Scholar] [CrossRef] [PubMed]
- Khamzina, L.; Veilleux, A.; Bergeron, S.; Marette, A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: Possible involvement in obesity-linked insulin resistance. Endocrinology 2005, 146, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; D’Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein s6 kinase 1, s6k1. Cell Metab. 2006, 3, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Layman, D.K. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J. Nutr. 1999, 129, 1102–1106. [Google Scholar] [PubMed]
- Lynch, C.J.; Patson, B.J.; Anthony, J.; Vaval, A.; Jefferson, L.S.; Vary, T.C. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E503–E513. [Google Scholar] [CrossRef] [PubMed]
- Shapira, J.F.; Kircher, I.; Martin, R.J. Indices of skeletal muscle growth in lean and obese zucker rats. J. Nutr. 1980, 110, 1313–1318. [Google Scholar] [PubMed]
- Cleary, M.P.; Vasselli, J.R. Reduced organ growth when hyperphagia is prevented in genetically obese (fa/fa) zucker rats. Proc. Soc. Exp. Biol. Med. 1981, 167, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Benton, C.R.; Han, X.X.; Febbraio, M.; Graham, T.E.; Bonen, A. Inverse relationship between pgc-1alpha protein expression and triacylglycerol accumulation in rodent skeletal muscle. J. Appl. Physiol. (1985) 2006, 100, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.M.; Mojtahedi, M.C.; Thorpe, M.P.; Valentine, R.J.; Kris-Etherton, P.M.; Layman, D.K. Effects of protein intake and gender on body composition changes: A randomized clinical weight loss trial. Nutr. Metab. (Lond.) 2012, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. Ain-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]



| L20 | L40 | O20 | O40 | Effect of Diet (20% v 40% Protein) | Effect of Body Weight (Lean v Obese) | Interaction Diet X Body Weight | |
|---|---|---|---|---|---|---|---|
| Alanine | 399 ± 26 | 327 ± 25 | 380 ± 39 | 381 ± 25 | ns | ns | ns |
| Glycine | 135 ± 8 a | 95 ± 4 b | 98 ± 5 b | 64 ± 3 c | <0.0001 | <0.0001 | ns |
| Valine | 167 ± 9 a | 194 ± 10 a,b | 161 ± 10 a | 232 ± 14 b | <0.001 | ns | ns |
| Leucine | 112 ± 6 a,b | 135 ± 5 a,c | 108 ± 7 b | 151 ± 9 c | <0.0001 | ns | ns |
| Isoleucine | 60 ± 3 a | 69 ± 2 a,b | 59 ± 4 a | 81 ± 5 b | <0.001 | ns | ns |
| Threonine | 277 ± 11 a,b | 230 ± 8 a,c | 309 ± 34 b | 187 ± 9 c | <0.0001 | ns | <0.05 |
| Serine | 163 ± 6 a,b | 151 ± 5 a,b | 168 ± 11 a | 142 ± 5 b | <0.01 | ns | ns |
| Proline | 259 ± 11 a, | 189 ± 11 b | 141 ± 13 c | 144 ± 10 c | <0.05 | <0.0001 | <0.01 |
| Asparagine | 35 ± 2 a | 30 ± 1 b | 28 ± 2 b | 25 ± 1 b | <0.01 | <0.001 | ns |
| Aspartate | 8.6 ± 0.6 | 8.2 ± 0.4 | 7.9 ± 0.6 | 7.9 ± 0.5 | ns | ns | ns |
| Methionine | 36 ± 2 a | 25 ± 2 b | 25 ± 3 b | 23 ± 1 b | <0.01 | <0.01 | <0.05 |
| Glutamate | 132 ± 8 a | 107 ± 4 a,b | 115 ± 7 a,b | 93 ± 7 b | <001 | <0.05 | ns |
| Phenylalanine | 52 ± 3 | 54 ± 3 | 56 ± 3 | 57 ± 3 | ns | ns | ns |
| Glutamine | 493 ± 36 a | 778 ± 73 b,d | 573 ± 38 b,c | 825 ± 36 d | <0.0001 | ns | ns |
| Lysine | 521 ± 40 a,b,c | 609 ± 17 a,c | 476 ± 33 b | 622 ± 31 c | <0.001 | ns | ns |
| Histidine | 44 ± 4 | 43 ± 3 | 49 ± 2 | 45 ± 2 | ns | ns | ns |
| Tyrosine | 68 ± 5 a,b | 53 ± 4 a | 74 ± 7 b | 52 ± 2 a | <0.001 | ns | ns |
| Tryptophan | 95 ± 6 a | 83 ± 4 a | 58 ± 5 b | 52 ± 4 b | <0.05 | <0.0001 | ns |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
French, W.W.; Dridi, S.; Shouse, S.A.; Wu, H.; Hawley, A.; Lee, S.-O.; Gu, X.; Baum, J.I. A High-Protein Diet Reduces Weight Gain, Decreases Food Intake, Decreases Liver Fat Deposition, and Improves Markers of Muscle Metabolism in Obese Zucker Rats. Nutrients 2017, 9, 587. https://doi.org/10.3390/nu9060587
French WW, Dridi S, Shouse SA, Wu H, Hawley A, Lee S-O, Gu X, Baum JI. A High-Protein Diet Reduces Weight Gain, Decreases Food Intake, Decreases Liver Fat Deposition, and Improves Markers of Muscle Metabolism in Obese Zucker Rats. Nutrients. 2017; 9(6):587. https://doi.org/10.3390/nu9060587
Chicago/Turabian StyleFrench, William W., Sami Dridi, Stephanie A. Shouse, Hexirui Wu, Aubree Hawley, Sun-Ok Lee, Xuan Gu, and Jamie I. Baum. 2017. "A High-Protein Diet Reduces Weight Gain, Decreases Food Intake, Decreases Liver Fat Deposition, and Improves Markers of Muscle Metabolism in Obese Zucker Rats" Nutrients 9, no. 6: 587. https://doi.org/10.3390/nu9060587





