(−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway
Abstract
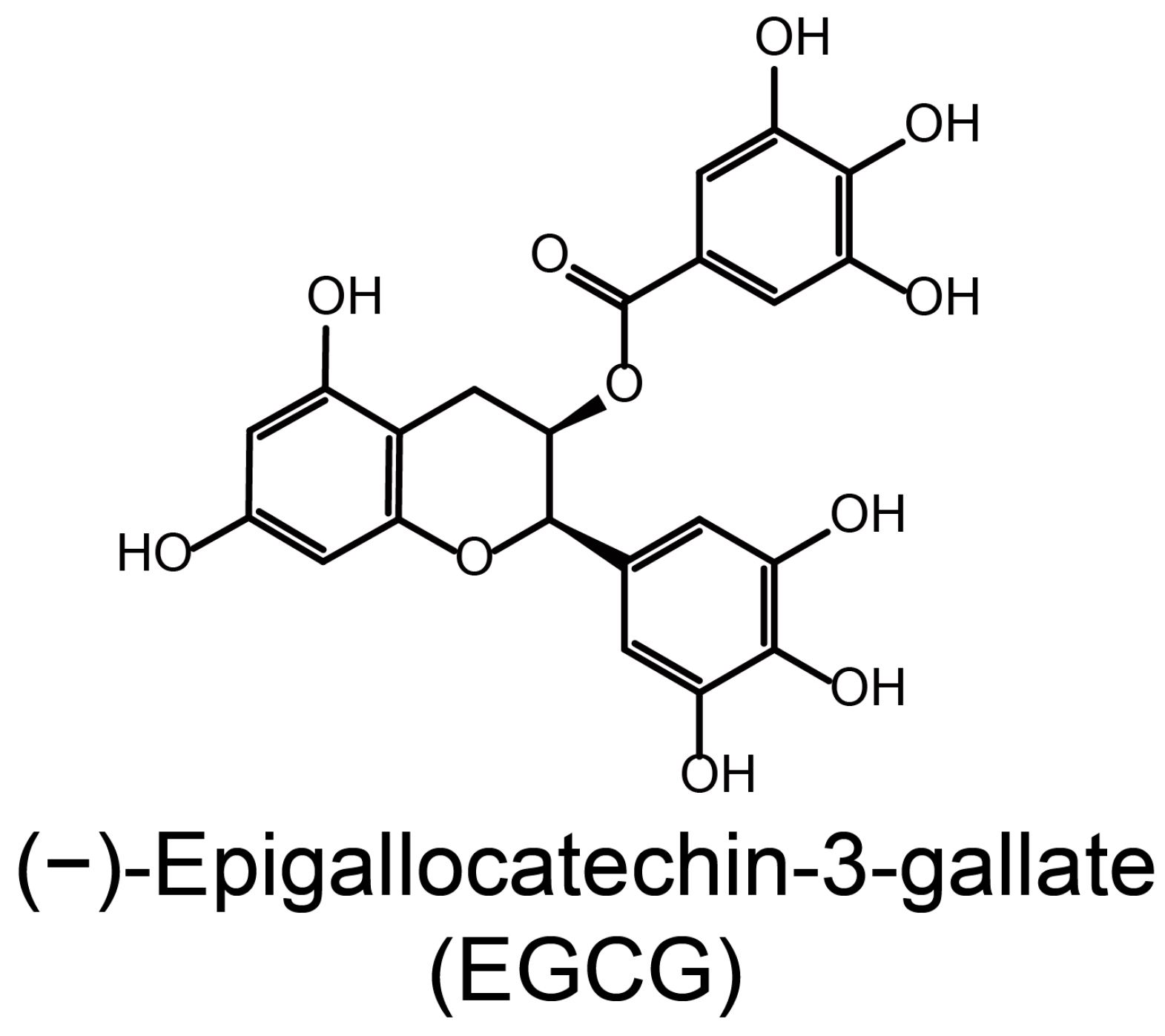
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Spheroid Formation Assay
2.3. Immunoblotting Analysis
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
- CD133-F, 5′-TACAACGCCAAACCACGACTGT-3′;
- CD133-R, 5′-TCTGAACCAATGGAATTCAAGACCCTTT-3′;
- CD44-F, 5′-GACACATATTGTTTCAATGCTTCAGC-3′;
- CD44-R, 5′-GATGCCAAGATGATCAGCCATTCTGGAAT-3′;
- ALDHA1-F, 5′-GCACGCCAGACTTACCTGTC-3′;
- ALDHA1-R, 5′-CCTCCTCAGTTGCAGGATTAAAG-3′;
- Oct4-F, 5′-TGGGATATACACAGGCCGATG-3′;
- Oct4-R, 5′-TCCTCCACCCACTTCTGAG-3′;
- Nanog-F, 5′-TTTGTGGGCCTGAAGAAAACT-3′;
- Nanog-R, 5′-AGGGCTGTCCTGAATAAGCAG-3′;
- β-catenin-F, 5′-AAGACATCACTGAGCCTCCAT-3′;
- β-catenin-R, 5′-CGATTTGCGGGACAAAGGGCAA-3′;
- PCNA-F, 5′-CTGAAGCCGAAACAGCTAGACT-3′;
- PCNA-R, 5′-TCGTTGATGAGGTCTTGAGTGC-3′;
- Cyclin D1-F, 5′-AGGCCCTGGCTGCTACAAG-3′;
- Cyclin D1-R, 5′-ACATCTGAGTGGGTCTGGAG-3′;
- GAPDH-F, 5′-CAAGGTCACCATGACAACTTTG-3′;
- GAPDH-R, 5′-GTCCACCACCCTGTTGCTGTAG-3′.
2.5. Immunofluorescence Staining
2.6. Statistical Analysis
3. Results
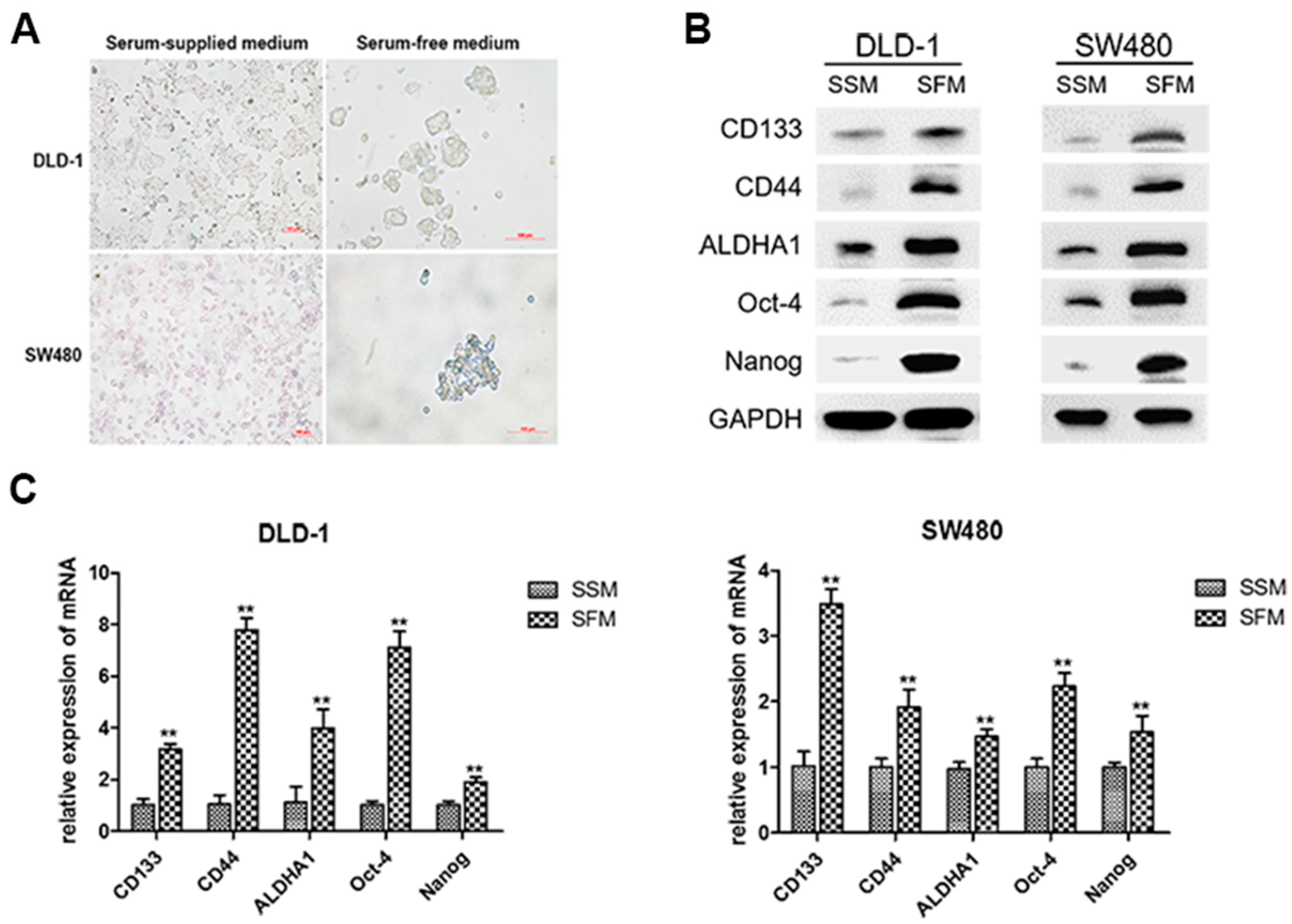
3.1. Enrichment of Colorectal CSCs by Serum-Free-Medium Culture
3.2. EGCG Inhibited the CSCs Properties and Wnt/β-Catenin Pathway in Colorectal CSCs
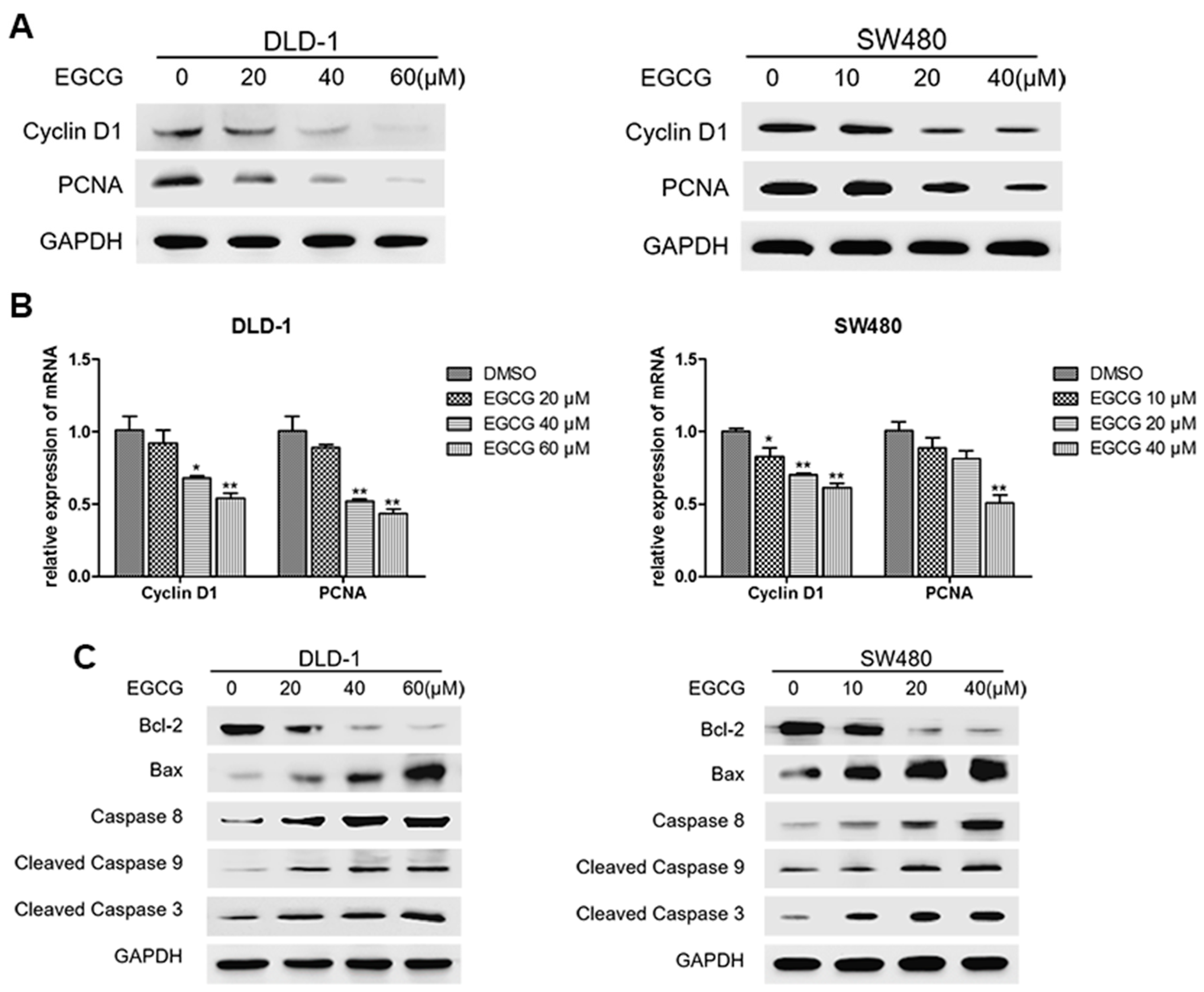
3.3. EGCG Reduced Cell Proliferation and Induced Apoptosis of Colorectal CSCs
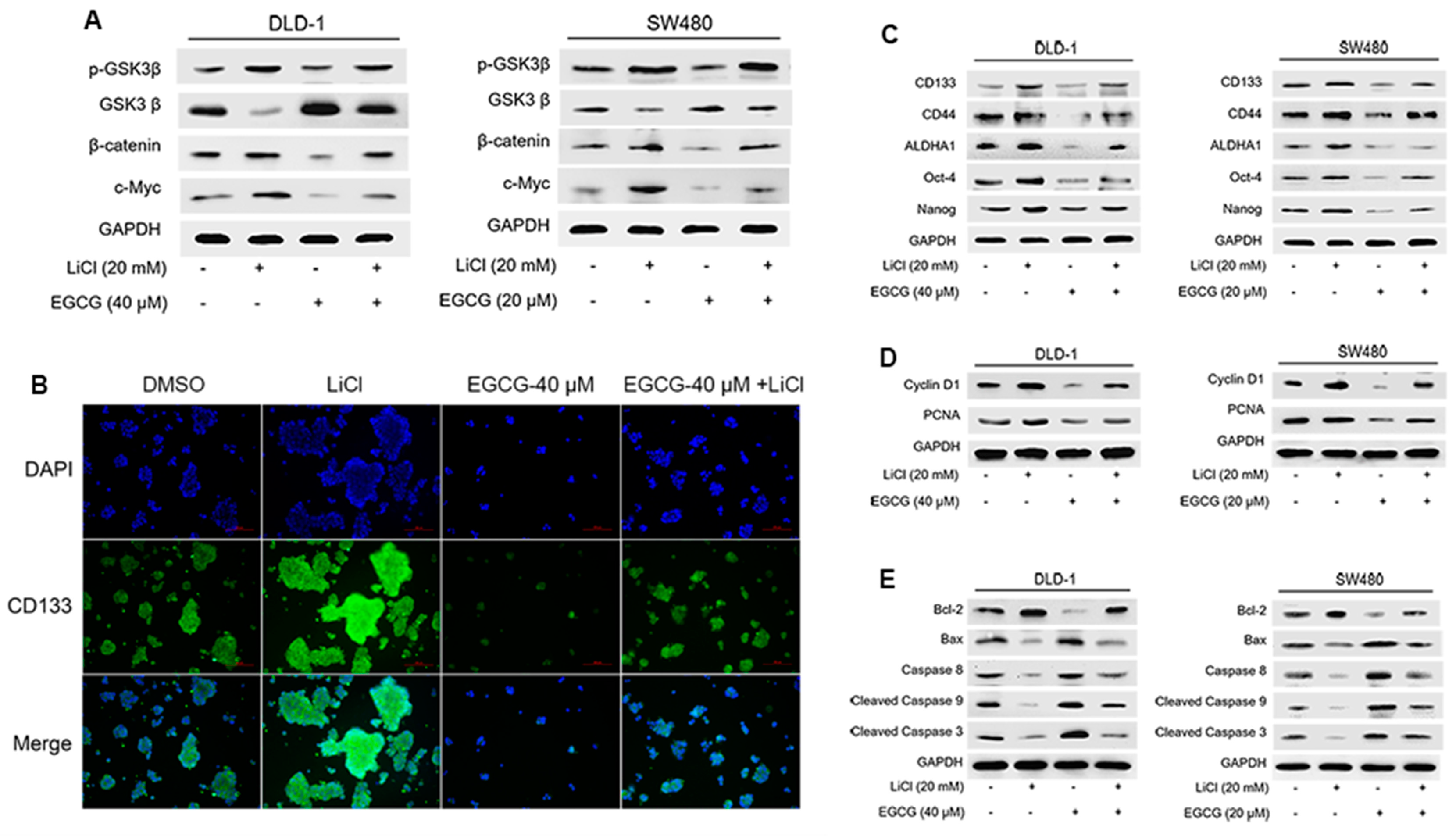
3.4. EGCG Diminished Colorectal CSCs Properties through Suppression of Wnt/β-Catenin Pathway
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cunningham, D.; Atkin, W.; Lenz, H.J.; Lynch, H.T.; Minsky, B.; Nordlinger, B.; Starling, N. Colorectal cancer. Lancet 2010, 375, 1030–1047. [Google Scholar] [CrossRef]
- Mettu, N.B.; Hurwitz, H.; Hsu, D.S. Use of molecular biomarkers to inform adjuvant therapy for colon cancer. Oncology 2013, 27, 746–754. [Google Scholar] [PubMed]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2006, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013, 341, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Francipane, M.G.; Medema, J.P.; Stassi, G. Colon Cancer Stem Cells: Promise of Targeted Therapy. Gastroenterology 2010, 138, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal cancer stem cells: From the crypt to the clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Cai, D.L.; Sun, F.; Wu, Z.H.; Yue, B.; Zhao, S.L.; Wu, X.S.; Zhang, M.; Zhu, X.W.; Peng, Z.H.; et al. FERMT1 mediates epithelial-mesenchymal transition to promote colon cancer metastasis via modulation of beta-catenin transcriptional activity. Oncogene 2016. [Google Scholar] [CrossRef]
- Rao, T.P.; Kuhl, M. An Updated Overview on Wnt Signaling Pathways: A Prelude for More. Circ. Res. 2010, 106, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Pradhan, S.J.; Patil, P.; Mulherkar, R.; Galande, S. Wnt/beta-catenin signaling regulated SATB1 promotes colorectal cancer tumorigenesis and progression. Oncogene 2016, 35, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, A.; Ye, J.; Zheng, X.; Polito, C.; Lu, J.; Li, Q.; Liang, Y. Suppressive Effects of Tea Catechins on Breast Cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wu, C.; Hsu, H.; Chuang, H.; Huang, S.; Tsai, C.; Chang, Y.; Tsao, G.; Chen, C.; Chen, J. EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by Up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2015, 16, 2530–2558. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. 2015, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wei, F.; Wang, Y.; Wu, B.; Fang, Y.; Xiong, B. EGCG synergizes the therapeutic effect of cisplatin and oxaliplatin through autophagic pathway in human colorectal cancer cells. J. Pharmacol. Sci. 2015, 128, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/beta-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Rajendra, G.S.; Levi, E.; Majumdar, A.P. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol. Cancer 2015, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; Ramalingam, S.; Houchen, C.W.; Anant, S. Cancer stem cells: A novel paradigm for cancer prevention and treatment. Mini Rev. Med. Chem. 2010, 10, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Christodoulou, M.S.; Silvani, A.; Herold-Mende, C.; Passarella, D. Chemical approaches to targeting drug resistance in cancer stem cells. Drug Discov. Today 2014, 19, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Marucci, C.; Fumagalli, G.; Calogero, F.; Silvani, A.; Christodoulou, M.S.; Martinet, N.; Passarella, D. Natural Products and Cancer Stem Cells. Curr. Pharm. Des. 2015, 21, 5547–5557. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, J.; Srinivasan, S.; Ankem, M.K.; Damodaran, C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015, 35, 5773–5788. [Google Scholar] [PubMed]
- Li, S.; Fu, J.; Watkins, D.N.; Srivastava, R.K.; Shankar, S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog–GLI pathway. Mol. Cell. Biochem. 2013, 373, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014, 346, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Yang, X.; Chen, Y.; Jiang, Y.; Wang, S.J.; Li, Y.; Wang, X.Q.; Meng, Y.; Zhu, M.M.; Ma, X.; et al. Curcumin Suppresses Lung Cancer Stem Cells via Inhibiting Wnt/beta-catenin and Sonic Hedgehog Pathways. Phytother. Res. 2017, 31, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Seino, M.; Okada, M.; Shibuya, K.; Seino, S.; Suzuki, S.; Takeda, H.; Ohta, T.; Kurachi, H.; Kitanaka, C. Differential Contribution of ROS to Resveratrol-induced Cell Death and Loss of Self-renewal Capacity of Ovarian Cancer Stem Cells. Anticancer Res. 2015, 35, 85–96. [Google Scholar] [PubMed]
- Cao, C.; Sun, L.; Mo, W.; Sun, L.; Luo, J.; Yang, Z.; Ran, Y. Quercetin Mediates beta-Catenin in Pancreatic Cancer Stem-Like Cells. Pancreas 2015, 44, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Wang, S.; Sang, Y.; Sun, P.; Lal, B.; Goodwin, C.R.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Laterra, J.; Xia, S. Regulation of glioblastoma stem cells by retinoic acid: Role for Notch pathway inhibition. Oncogene 2011, 30, 3454–3467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, Y.; Li, H.; Qiu, D.; Wang, Z.; Tan, S. Inhibition of Cell Survival by Curcumin Is Associated with Downregulation of Cell Division Cycle 20 (Cdc20) in Pancreatic Cancer Cells. Nutrients 2017, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M. Cancer prevention by green tea: Evidence from epidemiologic studies. Am. J. Clin. Nutr. 2013, 98, 1676S–1681S. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Chen, W.; Lung, W.; Wei, X.; Cheng, B.; Cai, Z.; Huang, W. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-kappa B and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-gallate(EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget 2016, 7, 79543–79557. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.J.; Gu, Q.H.; Cao, L.M.; Yang, H.P.; Hu, C.P. EGCG Regulated Ku70 Acetylation for Apoptosis in Human Lung Cancer A549 Cells. J. Thorac. Oncol. 2015, 102, S487–S488. [Google Scholar]
- Lee, J.C.; Chung, L.C.; Chen, Y.J.; Feng, T.H.; Chen, W.T.; Juang, H.H. Upregulation of B-cell translocation gene 2 by epigallocatechin-3-gallate via p38 and ERK signaling blocks cell proliferation in human oral squamous cell carcinoma cells. Cancer Lett. 2015, 360, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martin-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J. Gastroen. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef] [PubMed]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.; Weissman, I.L.; Passegue, E. Chronic versus acute myelogenous leukemia: A question of self-renewal. Cancer Cell 2004, 6, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Bisson, I.; Prowse, D.M. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009, 19, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, S.; Yoon, Y. The anti-adipogenic effects of (−)epigallocatechin gallate are dependent on the WNT/β-catenin pathway. J. Nutr. Biochem. 2013, 24, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, X.-Q.; Zhang, Q.; Zhu, J.-Y.; Li, Y.; Xie, C.-F.; Li, X.-T.; Wu, J.-S.; Geng, S.-S.; Zhong, C.-Y.; et al. (−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. https://doi.org/10.3390/nu9060572
Chen Y, Wang X-Q, Zhang Q, Zhu J-Y, Li Y, Xie C-F, Li X-T, Wu J-S, Geng S-S, Zhong C-Y, et al. (−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients. 2017; 9(6):572. https://doi.org/10.3390/nu9060572
Chicago/Turabian StyleChen, Yue, Xiao-Qian Wang, Qi Zhang, Jian-Yun Zhu, Yuan Li, Chun-Feng Xie, Xiao-Ting Li, Jie-Shu Wu, Shan-Shan Geng, Cai-Yun Zhong, and et al. 2017. "(−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway" Nutrients 9, no. 6: 572. https://doi.org/10.3390/nu9060572





