Nutritional Vitamin D in Renal Transplant Patients: Speculations and Reality
Abstract
:1. Introductory Notes
2. Limitations in the Assessment of Vitamin D Status
3. Epidemiology of the Vitamin D Status in KTx Recipients
4. Vitamin D Status and Mineral and Bone Disease in KTx
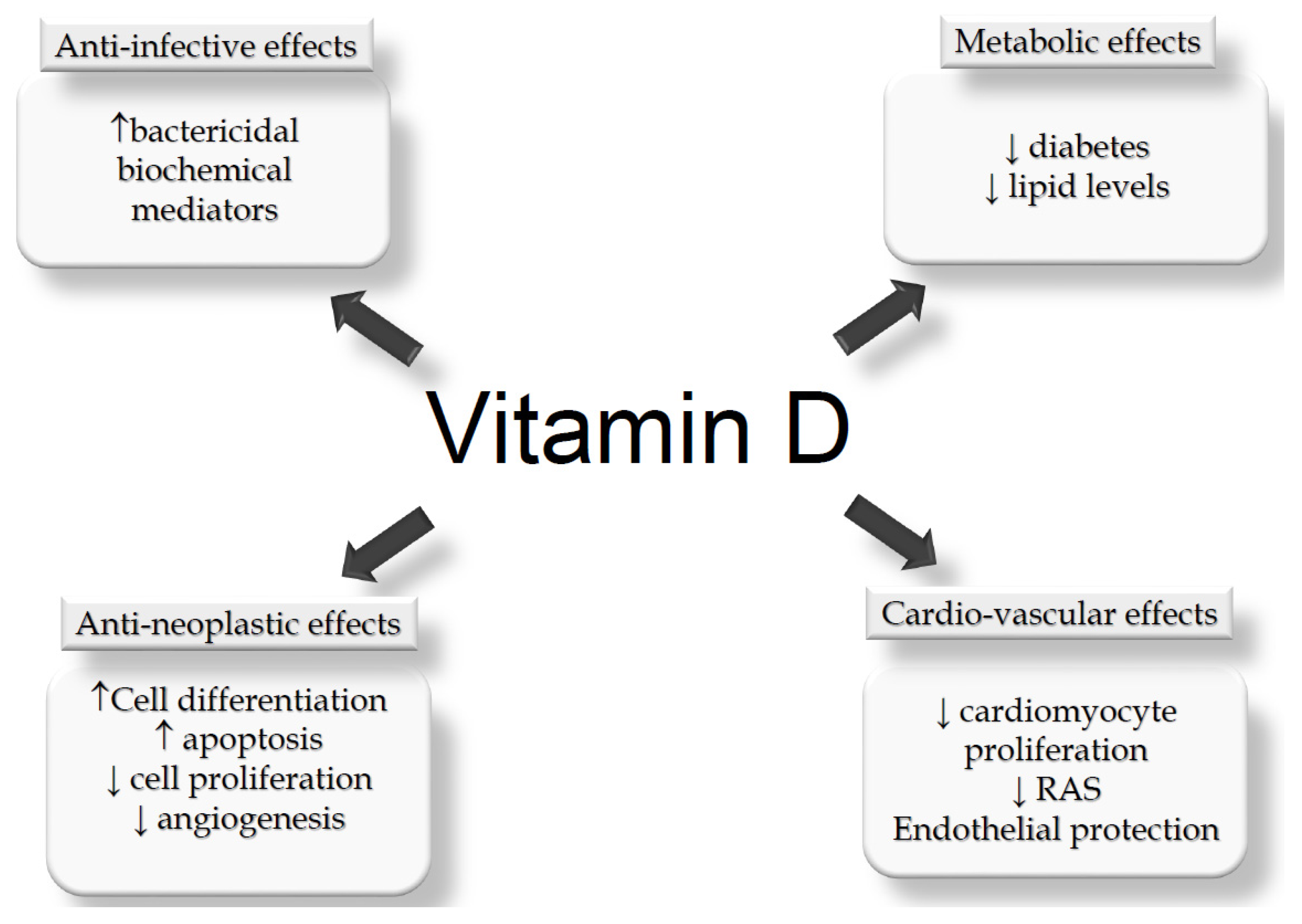
5. Vitamin D Status and Potential Non-MBM Related Effects in KTx
5.1. Potential Effects Counteracting KTx Complications
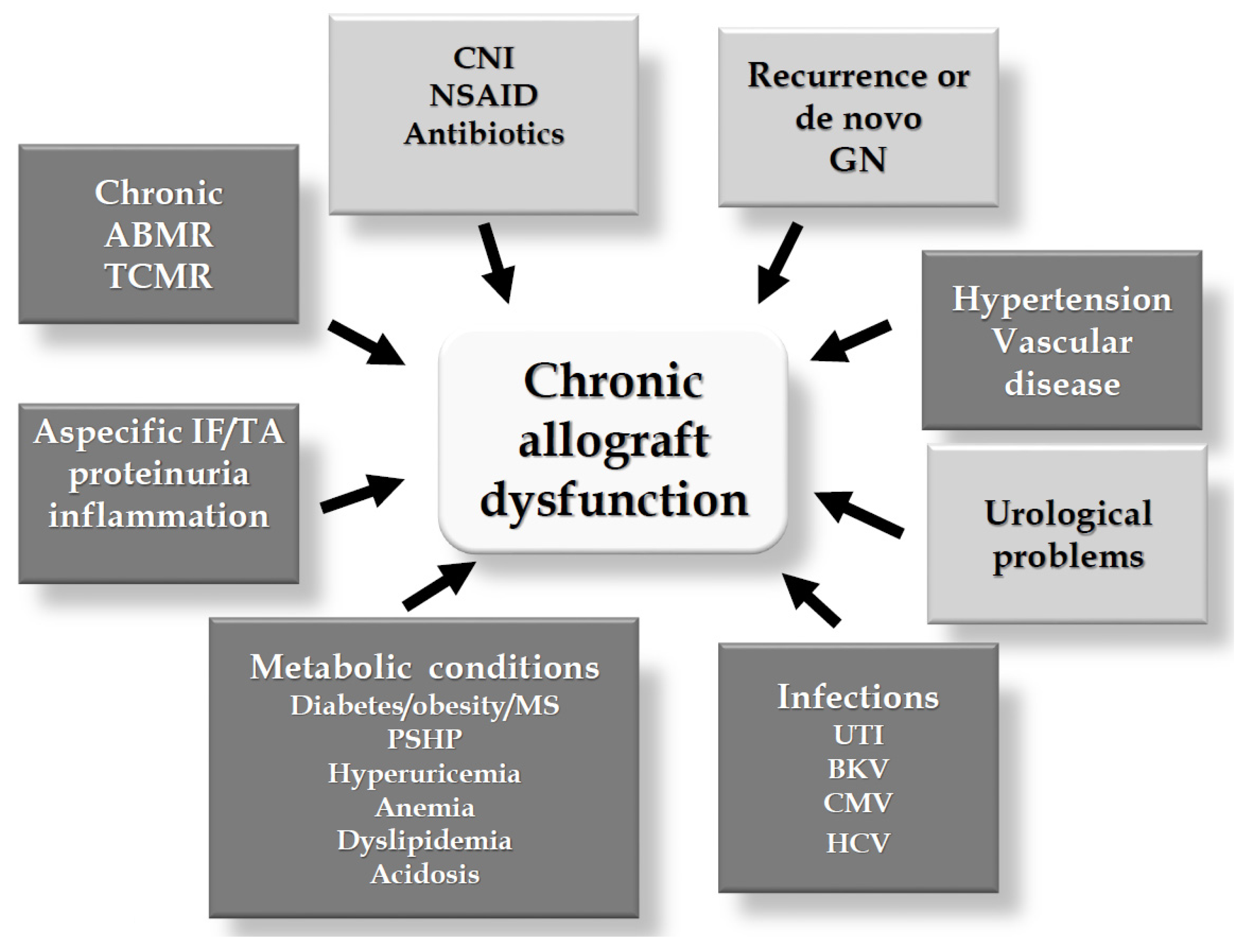
5.2. Potential Effects Counteracting Allograft Dysfunction
6. Conclusive Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeLuca, H.F. Vitamin D: Historical Overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, E319. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008, 66, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Alfieri, C.; Rastaldi, M.P. Recent insights into vitamin D and its receptor. J. Nephrol. 2011, 24, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.; Brown, A.; Slatopolsky, E. Extrarenal production of calcitriol. Semin. Nephrol. 1994, 14, 144–155. [Google Scholar] [PubMed]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; Iida, S.; Tyson, J.H.; Turner, A.G.; Morris, H.A. Bone CYP27B1 gene expression is increased with high dietary calcium and in mineralising osteoblasts. J. Steroid Biochem. Mol. Biol. 2010, 121, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.A.; Anderson, P.H. Autocrine and paracrine actions of vitamin D. Clin. Biochem. Rev. 2010, 31, 129–138. [Google Scholar] [PubMed]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H. Vitamin D and glucose metabolism in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2008, 17, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; McCann, M.; Zhang, Z.; Posner, G.H.; Bingham, V.; El-Tanani, M.; Campbell, F.C. Vitamin D receptor modulates the neoplastic phenotype through antagonistic growth regulatory signals. Mol. Carcinog. 2009, 48, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Curreri, M.; Regalia, A.; Alfieri, C.M. Vitamin D and the cardiovascular system: An overview of the recent literature. Am. J. Cardiovasc. Drugs 2014, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Tjønneland, A.; Olsen, A.; Halkjær, J.; Lind, B.; Heegaard, A.M.; Schwarz, P. A Reverse J-Shaped Association between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J. Clin. Endocrinol. Metab. 2015, 100, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Krueger, D.; Lensmeyer, G. 25-Hydroxyvitamin D measurement 2009: A review for clinicians. J. Clin. Densitom. 2009, 12, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.J.; Martin, S.; McWhinney, B.; Straub, I.; Williams, P.; Herrmann, M. State-of-the-art vitamin D assays: A comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin. Chem. 2012, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.D.; Milan, A.M. Vitamin D assays: Past and present debates, difficulties, and developments. Calcif. Tissue Int. 2013, 92, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.I.; Register, T.C. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nat. Rev. Nephrol. 2012, 8, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.P.; Robinson-Cohen, C.; de Boer, I.H.; Houston, D.K.; Lohman, K.; Liu, Y.; Kritchevsky, S.B.; Cauley, J.A.; Tanaka, T.; Ferrucci, L.; et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012, 308, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.M.; Lucas, R.M.; Harrison, S.L.; van der Mei, I.A.; Armstrong, B.; Kricker, A.; Mason, R.S.; McMichael, A.J.; Nowak, M.; Whiteman, D.C.; et al. The AusD Study: A population-based study of the determinants of serum 25-hydroxyvitamin D concentration across a broad latitude range. Am. J. Epidemiol. 2013, 177, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Denburg, M.R.; Hoofnagle, A.N.; Sayed, S.; Gupta, J.; de Boer, I.H.; Appel, L.J.; Durazo-Arvizu, R.; Whitehead, K.; Feldman, H.I.; Leonard, M.B.; et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations across Genotypes. J. Bone Miner. Res. 2016, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J. Bone Miner. Res. 2011, 26, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Van Schoor, N.M.; Gielen, E.; Boonen, S.; Mathieu, C.; Vanderschueren, D.; Lips, P. Optimal vitamin D status: A critical analysis on the basis of evidence-based medicine. J. Clin. Endocrinol. Metab. 2013, 98, e1283–e1304. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.; Houillier, P.; Gauci, C.; Haymann, J.P.; Flamant, M.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease: Quest for a threshold. J. Clin. Endocrinol. Metab. 2013, 98, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes: Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Messa, P.; Sindici, C.; Cannella, G.; Miotti, V.; Risaliti, A.; Gropuzzo, M.; Di Loreto, P.L.; Bresadola, F.; Mioni, G. Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int. 1998, 54, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Shane, E. Vitamin D in organ transplantation. Osteoporos. Int. 2011, 22, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Querings, K.; Girndt, M.; Geisel, J.; Georg, T.; Tilgen, W.; Reichrath, J. 25-hydroxyvitamin D deficiency in renal transplant recipients. J. Clin. Endocrinol. Metab. 2006, 91, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Boudville, N.C.; Hodsman, A.B. Renal function and 25-hydroxyvitamin D concentrations predict parathyroid hormone levels in renal transplant patients. Nephrol. Dial. Transplant. 2006, 21, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Sadlier, D.M.; Magee, C.C. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: A prospective study. Clin. Transplant. 2007, 21, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Stavroulopoulos, A.; Cassidy, M.J.; Porter, C.J.; Hosking, D.J.; Roe, S.D. Vitamin D status in renal transplant recipients. Am. J. Transplant. 2007, 7, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Ewers, B.; Gasbjerg, A.; Moelgaard, C.; Frederiksen, A.M.; Marckmann, P. Vitamin D status in kidney transplant patients: Need for intensified routine supplementation. Am. J. Clin. Nutr. 2008, 87, 431–437. [Google Scholar] [PubMed]
- Mazzaferro, S.; Pasquali, M.; Pugliese, F.; Citterio, F.; Gargiulo, A.; Rotondi, S.; Tartaglione, L.; Conte, C.; Pirrò, G.; Taggi, F. Distinct impact of vitamin D insufficiency on calcitriol levels in chronic renal failure and renal transplant patients: A role for FGF23. J. Nephrol. 2012, 25, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Marcén, R.; Jimenez, S.; Fernández-Rodriguez, A.; Galeano, C.; Villafruela, J.J.; Gomis, A.; Teruel, J.L.; Quereda, C. Are low levels of 25-hydroxyvitamin D a risk factor for cardiovascular diseases or malignancies in renal transplantation? Nephrol. Dial. Transplant. 2012, 27, iv47–iv52. [Google Scholar] [CrossRef] [PubMed]
- Bienaimè, F.; Girard, D.; Anglicheau, D.; Canaud, G.; Souberbielle, J.C.; Kreis, H.; Noël, L.H.; Friedlander, G.; Elie, C.; Legendre, C.; et al. Vitamin D status and outcomes after renal transplantation. J. Am. Soc. Nephrol. 2013, 24, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Beique, L.C.; Kline, G.A.; Dalton, B.; Duggan, K.; Yilmaz, S. Predicting deficiency of vitamin D in renal transplant recipients in northern climates. Transplantation 2013, 95, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Dadhania, D.; August, P.; Lee, J.B.; Suthanthiran, M.; Muthukumar, T. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation 2014, 98, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Hamano, T.; Ichimaru, N.; Tomida, K.; Matsui, I.; Fujii, N.; Okumi, M.; Kaimori, J.Y.; Yazawa, K.; Kokado, Y.; et al. Vitamin D deficiency predicts decline in kidney allograft function: A prospective cohort study. J. Clin. Endocrinol. Metab. 2014, 99, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, A.; Fournier, M.C.; llaizeau, F.; Masson, D.; Giral, M.; Cariou, B.; Cantarovic, D.; Dantal, J. Vitamin D deficiency is an independent risk factor for PTDM after kidney transplantation. Transpl. Int. 2016, 29, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; Riphagen, I.J.; Joosten, M.M.; Navis, G.; Muller Kobold, A.C.; Kema, I.P.; Bakker, S.J.; de Borst, M.H.; NIGRAM Consortium. Associations of 25(OH) and 1,25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J. Clin. Endocrinol. Metab. 2015, 100, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Filipov, J.J.; Zlatkov, B.K.; Dimitrov, E.P.; Svinarov, D.A. Higher 25-Hydroxyvitamin D Levels are Associated with Lower Proteinuria in Kidney Transplant Recipients. Exp. Clin. Transplant. 2016, 14, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Doorenbos, C.R.; de Cuba, M.M.; Vogt, L.; Kema, I.P.; van den Born, J.; Gans, R.O.; Navis, G.; de Borst, M.H. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J. Steroid Biochem. Mol. Biol. 2012, 128, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Cafforio, C.; Alfieri, C. Calcium and phosphate changes after renal transplantation. J. Nephrol. 2010, 23, S175–S181. [Google Scholar] [PubMed]
- Evenepoel, P.; Behets, G.J.; Viaene, L.; D’Haese, P.C. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int. 2017, 91, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Sella, S.; Silva Netto, F.; Cattelan, C.; Dalle Carbonare, L.; Lazzarin, R.; Marchini, F.; Rigotti, P.; Marcocci, C.; Cetani, F.; et al. Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: Role of calcium-sensing receptor polymorphisms and vitamin D deficiency. J. Bone Miner. Res. 2010, 25, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin. Nephrol. 2013, 33, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Cafforio, C.; Alfieri, C. Clinical impact of hypercalcemia in kidney transplant. Int. J. Nephrol. 2011, 2011, 906832. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, M.; Regalia, A.; Alfieri, C.; Barretta, F.; Croci, D.; Gandolfo, M.T.; Vettoretti, S.; Rastaldi, M.P.; Messa, P. Calcium and osteoprotegerin levels predict the progression of the abdominal aortic calcifications after kidney transplantation. Transplantation 2013, 96, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Wall, B.M.; Cooke, R. Osteomalacia and secondary hyperparathyroidism after kidney transplantation: Relationship to vitamin D deficiency. Am. J. Med. Sci. 2007, 333, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Coates, P.S.; Russ, G.R.; Coates, P.T. Hyperparathyroidism and vitamin D deficiency predispose to bone loss in renal transplant recipients. Transplantation 2009, 88, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; McGregor, D.O.; Strippoli, G.F. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst. Rev. 2007, CD005015. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Molnar, M.Z.; Kovesdy, C.P.; Mucsi, I.; Bunnapradist, S. Management of mineral and bone disorder after kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2012, 21, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Thervet, E.; Souberbielle, J.C.; Zuber, J.; Eladari, D.; Martinez, F.; Mamzer-Bruneel, M.F.; Urena, P.; Legendre, C.; Friedlander, G.; et al. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009, 75, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Barros, X.; Rodríguez, N.Y.; Fuster, D.; Rodas, L.; Esforzado, N.; Mazza, A.; Rubello, D.; Campos, F.; Tapias, A.; Torregrosa, J.V. Comparison of two different vitamin D supplementation regimens with oral calcifediol in kidney transplant patients. J. Nephrol. 2016, 29, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Wissing, K.M.; Broeders, N.; Moreno-Reyes, R.; Gervy, C.; Stallenberg, B.; Abramowicz, D. A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation 2005, 79, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Kiener, C.; Javier, R.M.; Braun, L.; Cognard, N.; Gautier-Vargas, G.; Heibel, F.; Muller, C.; Olagne, J.; Moulin, B.; et al. Recent changes in chronic kidney disease-mineral and bone disorders (CKD-MBD) and associated fractures after kidney transplantation. Transplantation 2016. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.; Li, G.; Penny, H.; Lombardi, G.; Afzali, B.; Goldsmith, D.J. Vitamin D in renal transplantation from biological mechanisms to clinical benefits. Am. J. Transplant. 2014, 14, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Rech, M.A.; Fleming, J.N.; Moore, C.L. 25-hydroxyvitamin D deficiency and opportunistic viral infections after kidney transplant. Exp. Clin. Transplant. 2014, 12, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Courivaud, C.; Bamoulid, J.; Kazory, A.; Dumoulin, G.; Chalopin, J.M. Pretransplant serum vitamin D levels and risk of cancer after renal transplantation. Transplantation 2008, 85, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The effect of vitamin D on renin-angiotensin system activation and blood pressure: A randomized control trial. J. Hypertens. 2017, 35, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Exner, D.V.; Hemmelgarn, B.R.; Hanley, D.A.; Turin, T.C.; MacRae, J.M.; Wheeler, D.C.; Sola, D.Y.; Ramesh, S.; Ahmed, S.B. The VITAH Trial-Vitamin D Supplementation and Cardiac Autonomic Tone in Patients with End-Stage Kidney Disease on Hemodialysis: A Blinded, Randomized Controlled Trial. Nutrients 2016, 8, E608. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grübler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Juraschek, S.P.; Bertenthal, M.S.; Detrick, B.; Furth, S.L.; Miller, E.R., 3rd. Pilot study of the effect of cholecalciferol supplementation on hepcidin in children with chronic kidney disease: Results of the D-fense Trial. Pediatr. Nephrol. 2017, 32, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Zheng, C.M.; Lu, C.L.; Lin, Y.F.; Shyu, J.F.; Wu, C.C.; Lu, K.C. Vitamin D and immune function in chronic kidney disease. Clin. Chim. Acta 2015, 450, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Széles, L.; Keresztes, G.; Töröcsik, D.; Balajthy, Z.; Krenács, L.; Póliska, S.; Steinmeyer, A.; Zuegel, U.; Pruenster, M.; Rot, A.; et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009, 182, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Sterling, K.A.; Eftekhari, P.; Girndt, M.; Kimmel, P.L.; Raj, D.S. The immunoregulatory function of vitamin D: Implications in chronic kidney disease. Nat. Rev. Nephrol. 2012, 8, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Morán-Auth, Y.; Penna-Martinez, M.; Shoghi, F.; Ramos-Lopez, E.; Badenhoop, K.J. Vitamin D status and gene transcription in immune cells. Steroid Biochem. Mol. Biol. 2013, 136, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Xu-Dubois, Y.C.; Thervet, E.; Prié, D.; Zuber, J.; Kreis, H.; Legendre, C.; Rondeau, E.; Pallet, N. Cholecalciferol supplementation does not protect against renal allograft structural and functional deterioration: A retrospective study. Transplantation 2011, 91, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.W.; Yoo, T.H.; Kim, M.S.; Kim, S.I.; Kim, Y.S.; Choi, K.H. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: An observational study. BMC Nephrol. 2012, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Namir, Y.; Cohen, M.J.; Haviv, Y.S.; Slotki, I.; Shavit, L. Vitamin D levels, vitamin D supplementation, and prognosis in patients with chronic kidney disease. Clin. Nephrol. 2016, 86, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Alberti, C.; Colas, S.; Prié, D.; Souberbielle, J.C.; Treluyer, J.M.; Thervet, E. Vitamin D supplementation in renal transplant recipients (VITALE): A prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials 2014, 15, 430. [Google Scholar] [CrossRef] [PubMed]
- Thiem, U.; Heinze, G.; Segel, R.; Perkmann, T.; Kainberger, F.; Mühlbacher, F.; Hörl, W.; Borchhardt, K. VITA-D: Cholecalciferol substitution in vitamin D deficient kidney transplant recipients: A randomized, placebo-controlled study to evaluate the post-transplant outcome. Trials 2009, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.P.; Nikkel, L.E.; Nishiyama, K.K.; Dworakowski, E.; Cremers, S.; Zhang, C.; McMahon, D.J.; Boutroy, S.; Liu, X.S.; Ratner, L.E.; et al. Kidney transplantation with early corticosteroid withdrawal: Paradoxical effects at the central and peripheral skeleton. J. Am. Soc. Nephrol. 2014, 25, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Mishra, S.K.; Mithal, A. Vitamin D toxicity resulting from overzealous correction of vitamin D deficiency. Clin. Endocrinol. 2015, 83, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.; Baudoin, R.; Kaufmann, M.; Souberbielle, J.C.; Ryckewaert, A.; Vantyghem, M.C.; Eckart, P.; Bacchetta, J.; Deschenes, G.; Kesler-Roussey, G.; et al. CYP24A1 Mutations in a Cohort of Hypercalcemic Patients: Evidence for a Recessive Trait. Clin. J. Endocrinol. Metab. 2015, 100, e1343–e1352. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O. CYP24A1 loss of function: Clinical phenotype of monoallelic and biallelic mutations. J. Steroid Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
| References | N/tot | Gender M % | Ethnicity % | Age Years m ± sd or (R) or (IQR) | KTx Vintage Years m ± sd or (R) or (IQR) | Country | Vitamin D Status % of Patients According to 25(OH)D Levels (ng/mL) |
|---|---|---|---|---|---|---|---|
| [33] | 31/n.r. | 54.8 | n.s. | (R 10–75) | 7 (R 0.5–19) | Germany | 48.3% < 15 |
| 48.5% 15–30 | |||||||
| 3.2% > 30 | |||||||
| [34] | 419/n.r. | 62.8 | n.s. | 51.0 ± 15 | 7.2 ± 6.4 | Canada | 27.2% < 16 |
| 48.2% 16–30 | |||||||
| 24.5% > 30 | |||||||
| [35] | 112/134 | 64.0 | Cauc 64.3 | 51.6 ± 13.1 | Assessed at time of KTx | United States | 28.6% < 10 |
| AA 24.1 | 58.9% 10–29 | ||||||
| Other 11.6 | 24.5% > 30 | ||||||
| [36] | 244/320 | 61.9 | Cauc 95 | 46.1 (R 21–76) | Short term (N. 104) 0.28 (R 0.16–0.98) | United Kingdom | 68% < 16 |
| 29% 16–30 | |||||||
| Asian 3.7 | 3% > 30 | ||||||
| Long term (N. 140) 6.0 (R 1–24) | 51% < 16 | ||||||
| Black 1.3 | 43% 16–30 | ||||||
| 6% > 30 | |||||||
| [37] | 173/242 | 49.9 | Cauc 91 | 53.4 ± 11.7 | 7.4 (IQR 3.3–12.7) | Denmark | 51% < 16 |
| 29% 16–30 | |||||||
| Black 9 | |||||||
| 20% > 30 | |||||||
| [38] | 111/n.r. | 58.8 | n.s. | 50.5 ± 11.5 | 6.7 ± 5.1 | Italy | 69.1% ≤ 30 |
| 21.9% > 30 | |||||||
| [39] | 331/389 | 61.6 | n.s. | 52.2 ± 14.1 | n.r. | Spain | 28.7% < 16 |
| 48.6% 16–29 | |||||||
| 22.7% > 29 | |||||||
| [40] | 634/n.r. | 58.7 | n.s. | 48.3 ± 13.4 | n.r. | France | 54.9% < 15 |
| 36.8% 15-30 | |||||||
| 8.3% > 30 | |||||||
| [41] | 331/717 | 51.1 | Cauc 85.8 | 51 (IQR 41.5–60.2) | 6.7 (IQR 2.9–10.8) | Canada | 45.3% ≤ 30 |
| Other 14.2 | 54.7% > 30 | ||||||
| [42] | 351/1211 | 63 | AA 22 | 52.3 ± 13.6 | n.r. | United States | 61.5% ≤ 20 |
| Other 78 | 38.5% > 20 | ||||||
| [43] | 264/n.r. | 61.3 | n.s. | 49.0 ± 12.3 | 10.4 (R 2–18) | Japan | 24.2% < 12 |
| 44.7% 12–20 | |||||||
| 31.1% > 20 | |||||||
| [44] | 444/1083 | 60.6 | Cauc 89.2 | 50.9 ± 13.7 | 4.0 (R 0–11) | France | 19.8% < 10 |
| 59.5% 10–30 | |||||||
| Other 10.8 | |||||||
| 20.7% > 30 | |||||||
| [45] | 435/847 | 51 | n.s. | 52 ± 12 | 6.3 (IQR 3.1–11.7) | The Netherlands | 49% < 20 |
| 33% 20–30 | |||||||
| 18% > 30 |
| Factor | Characteristics | Type of Effect |
|---|---|---|
| Ethnicity | Afro-Americans | neg |
| Age | Elderly | neg |
| Gender | Women | neg |
| BMI | High | neg |
| Smoking | Yes | neg |
| Sun exposure | Yes | pos |
| Dietary intake/VitD supplements | Yes | pos |
| Diabetes | Yes | neg |
| Liver dysfunction | Yes | neg |
| Urinary protein | High | neg (?) |
| Time from KTx | Early | neg |
| Steroid doses | High | neg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messa, P.; Regalia, A.; Alfieri, C.M. Nutritional Vitamin D in Renal Transplant Patients: Speculations and Reality. Nutrients 2017, 9, 550. https://doi.org/10.3390/nu9060550
Messa P, Regalia A, Alfieri CM. Nutritional Vitamin D in Renal Transplant Patients: Speculations and Reality. Nutrients. 2017; 9(6):550. https://doi.org/10.3390/nu9060550
Chicago/Turabian StyleMessa, Piergiorgio, Anna Regalia, and Carlo Maria Alfieri. 2017. "Nutritional Vitamin D in Renal Transplant Patients: Speculations and Reality" Nutrients 9, no. 6: 550. https://doi.org/10.3390/nu9060550




