Selected Risk Nutritional Factors for Chemotherapy-Induced Polyneuropathy
Abstract
:1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cancernetwork. Available online: http://www.cancernetwork.com/oncology-nursing/chemotherapy-induced-peripheral-neuropathy-cancer-survivors (accessed on 5 April 2017).
- Cancer Supportive Survivorship Care. Available online: http://www.cancersupportivecare.com/nervepain.html (accessed on 5 April 2017).
- Chau, Y.P.; Chien, C.L.; Lu, K.S. The permeability of capillaries among the small granule-containing cells in rat superior cervical ganglia: And ultrastructural lanthanum tracer study. Histol. Histopathol. 1991, 6, 261–268. [Google Scholar] [PubMed]
- Chaudhry, V.; Rowinsky, E.K.; Sartorius, S.E.; Donehower, R.C.; Cornblath, D.R. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann. Neurol. 1994, 35, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, P.A.; Balmaceda, C.; Peterson, K.; Seidman, A.D.; Brasher, P.; DeAngelis, L.M. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J. Neuro Oncol. 1997, 35, 47–53. [Google Scholar] [CrossRef]
- Wampler, M.A.; Hamolsky, D.; Hamel, K.; Melisko, M.; Topp, K.S. Case report: Painful peripheral neuropathy following treatment with docetaxel for breast cancer. Clin. J. Oncol. Nurs. 2005, 9, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Up To Date. Available online: http://www.uptodate.com/contents/image?imageKey=ONC%2F67516&topicKey=ONC%2F2836&source=see_link&utdPopup=true (accessed on 5 April 2017).
- Watkins, S.M.; Griffin, J.P. High incidence of vincristine-induced neuropathy in lymphomas. Br. Med. J. 1978, 11, 610–612. [Google Scholar] [CrossRef]
- Van der Hoop, R.G.; van der Burg, M.E.; ten Bokkel Huinink, W.W.; van Houwelingen, C. Incidence of neuropathy in 395 patients with ovarian cancer treated with or without cisplatin. Cancer 1990, 66, 1697–1702. [Google Scholar] [CrossRef]
- Amptoulach, S.; Tsavaris, N. Neurotoxicity caused by the treatment with platinum analogues. Chemother. Res. Pract. 2011. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, V.; Chaudhry, M.; Crawford, T.O.; Simmons-O’Brien, E.; Griffin, J.W. Toxic neuropathy in patients with pre-existing neuropathy. Neurology 2003, 60, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Bogliun, G.; Marzorati, L.; Zincone, A.; Piatti, M.; Colombo, N.; Franchi, D.; La Presa, M.T.; Lissoni, A.; Buda, A.; et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann. Oncol. 2004, 15, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, Z.; Esfahani, A.; Djazayeri, A.; Djalali, M.; Golestan, B.; Ayromlou, H.; Hashemzade, S.; Asghari Jafarabadi, M.; Montazeri, V.; Keshavarz, S.A.; et al. Omega-3 fatty acids are protective against paclitaxel-induces peripheral neuropathy: A randomized double-blind placebo controlled trial. BMC Cancer 2012, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Schloss, J.M.; Colosimo, M.; Airey, C.; Masci, P.; Linnane, A.W.; Vitetta, L. A randomized, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing development of chemotherapy-induced peripheral neuropathy (CIPN). Support. Care Cancer 2017, 25, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chabas, J.F.; Alluin, O.; Rao, G.; Garcia, S.; Lavaut, M.N.; Risso, J.J.; Legre, R.; Magalon, G.; Khrestchatisky, M.; Marqueste, T.; et al. Vitamin D2 potentiates axon regeneration. J. Neurotrauma 2008, 25, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Schlotter, C.M.; Rossmanith, W.G.; Ulmer, H.; Staiger, H.; Villena, C. Neoadjuvant chemotherapy for breast cancer with weekly nab-paclitaxel by epirubicin and cyclofosfamide-results of a case series. In Vivo 2014, 28, 235–241. [Google Scholar] [PubMed]
- Michigan Neuropathy Screening Instrument. Available online: http://www.med.umich.edu/borc/profs/documents/svi/MNSI_patient.pdf (accessed on 5 April 2017).
- Cavaletti, G.; Bogliun, G.; Marzogatti, L.; Zincone, A.; Marzola, M.; Colombo, N.; Tredici, G. Peripheral toxicity of taxol in patients previously treated with cisplatin. Cancer 1995, 75, 1141–1150. [Google Scholar] [CrossRef]
- Schloss, J.; Colosimo, M.; Vitetta, L. New insights into potential prevention and management options for chemotherapy-induced peripheral neuropathy. Asia Pac. J. Oncol. Nurs. 2016, 3, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Eisenhauer, E.A.; Chaundry, V.; Arbuck, S.G.; Donehower, R.C. Clinical toxicities encountered with paclitaxel (TAXOL). Semin. Oncol. 1993, 4, 1–15. [Google Scholar]
- Powe, C.E.; Ricciardi, C.; Berg, A.H.; Erdenesanaa, D.; Collerone, G.; Ankers, E.; Wenger, J.; Karumanchi, S.A.; Thadhani, R.; Bhan, I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 2011, 26, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; De Meester, F. Omega-3 Fatty Acids in Brain and Neurological Health, 1st ed.; Elsevier: Waltham, MA, USA, 2014; p. 496. [Google Scholar]
| Chemotherapy Agent | Incidence of CIPN | Sensory Symptoms | Motor Symptoms | Recovery |
|---|---|---|---|---|
| Taxane Class | ||||
| Paclitaxel (Taxol®) | 50–70% | Mild distal loss of sensation to all modalities, feet greater than hands, painful paresthesias. Mild to moderate numbness, tingling, burning/stabbing pain of hands and feet are common, which can become severe with increased doses. | Weakness of distal muscles has been documented with high cumulative doses of paclitaxel and docetaxel. | Usually improves after treatment, but persistent symptoms in about 50% of patients one year later. |
| Docetaxel (Taxotere®) | 40–60% | |||
| Abraxane™ | 60–80% | |||
| Vinca Alkaloid Class | ||||
| Vincristine (Onkovin®) | 50–70% (1) | Distal sensory loss lower extremities, rarely affects upper extremities; vinblastine and vinorelbine are less neurotoxic; vincristine-rare mononeuropathies. | Weakness of distal muscles, decreased deep tendon reflexes, and foot drop have been noted with high doses. | Usually resolves within three months; may persist with vincristine. |
| Vinorelbine (Navelbine®) | 20–30% | |||
| Platinum Compounds | ||||
| Cisplatin (Platinol®) | 40–60% (2, 3) | Distal, symmetric loss of sensation to all modalities, stocking glove distribution; painful paresthesias or numbness. Symptoms can become severe with high cumulative doses. | Weakness is rare but can occur with high doses of cisplatin and oxaliplatin. | Partial; may progress for several months after drug is discontinued. |
| Carboplatin (Paraplatin®) | 2–8% | |||
| Oxaliplatin (Eloxatin®) | 70–80% | |||
| Characteristic | Number of Cases, %, or Mean ± SD |
|---|---|
| Total number of patients | 70 |
| Sex | 10% males; 90% females |
| Age (years) | 56 ± 12.2 |
| Body mass index (kg m−2) | 27.2 ± 5.5 |
| diabetes mellitus | 9.5% |
| tumor presence | Non 66%; neo-adjuvant 14%; palliative 8% |
| CIPN complication | 60% |
| Median 0 (25%; 75%) | Median 1 (25%; 75%) | Median 2 (25%; 75%) | p Value | |
|---|---|---|---|---|
| Score doctor | 0 (0; 0) | 0.2 (0.175; 0.2) | 0.3 (0.3; 0.4) | <0.05 *,**,*** |
| Score patient | 0 (0; 0) | 0.133 (0.133; 0.117) | 0.2 (0.2; 0.267) | <0.05 *,**,*** |
| Analyte | CIPN | Median | Mean ± SD | p Value |
|---|---|---|---|---|
| (Interquartile Range) | ||||
| Vitamin B1 (ng/mL) | 0 | 10.1 (6.38; 21.6) | - | ns |
| 1 | 9.72 (6.67; 14.1) | - | ||
| Vitamin B6 (ng/mL) | 0 | 6.65 (4.84; 14.3) | - | ns |
| 1 | 9.38 (4.6; 15.5) | - | ||
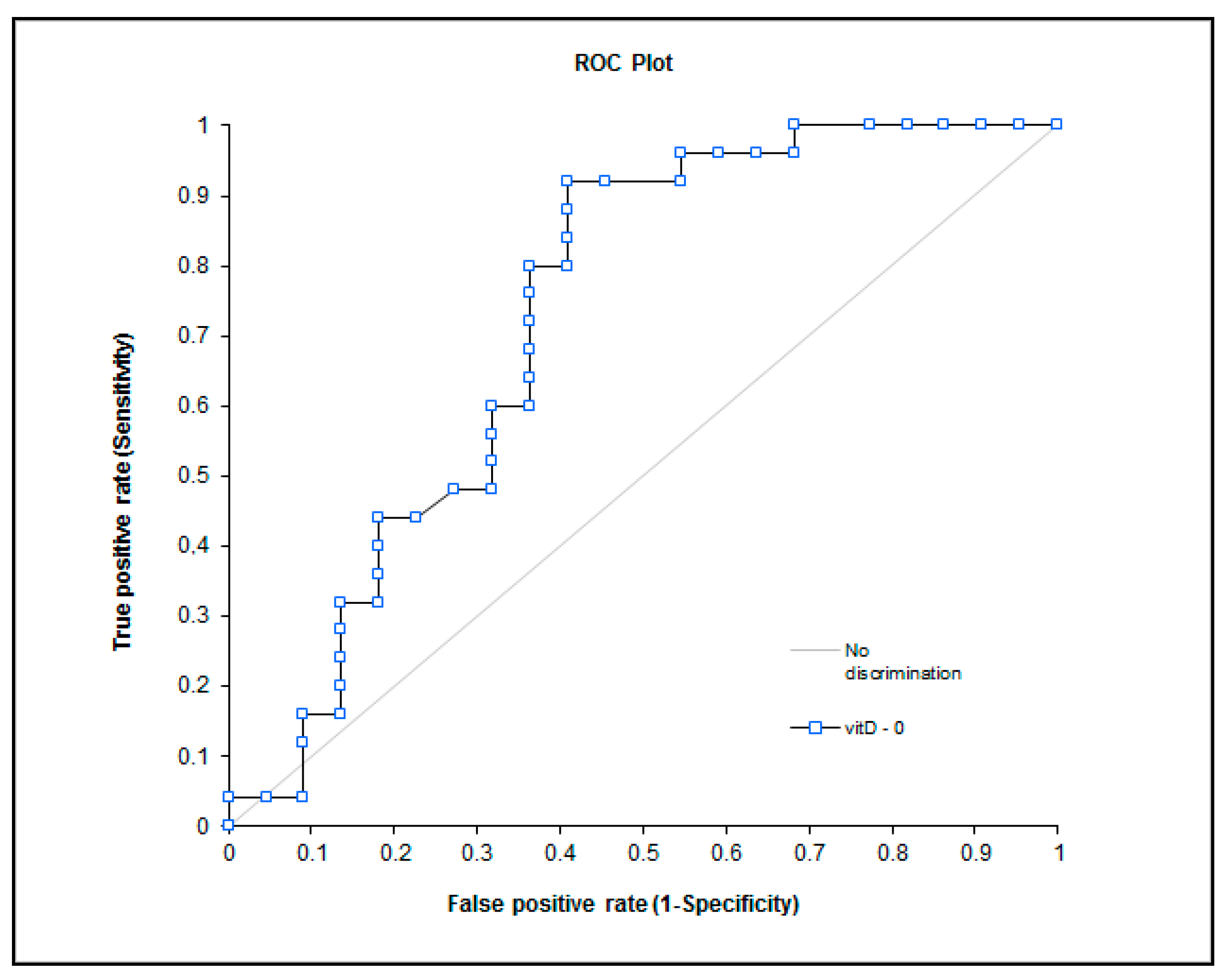
| Vitamin D (nmol/L) | 0 | 38.5 (24.95; 47.63) | - | 0.008 |
| 1 | 25.6 (19.7; 32.55) | - | ||
| C12:0 (Area %) | 0 | 0.18 (0.11; 0.31) | - | 0.031 |
| 1 | 0.24 (0.18; 0.42) | - | ||
| C14:0 (Area %) | 0 | 1.42 (1.02; 1.94) | - | 0.041 |
| 1 | 1.62 (1.34; 2.3) | - | ||
| C16:0 (Area %) | 0 | 24.34 (23.06; 27.23) | - | ns |
| 1 | 24.96 (23.7; 29.25) | - | ||
| C16:1 (Area %) | 0 | - | 2.42 ± 0.77 | 0.03 |
| 1 | - | 2.96 ± 1.11 | ||
| C18:0 (Area %) | 0 | - | 7.03 ± 0.65 | ns |
| 1 | - | 7.24 ± 0.69 | ||
| C18:1 (Area %) | 0 | - | 24.65 ± 3 | ns |
| 1 | - | 24.19 ± 2.64 | ||
| C18:2 n6 (Area %) | 0 | - | 27.36 ± 3.9 | ns |
| 1 | - | 26.19 ± 3.73 | ||
| C18:3 n6 (Area %) | 0 | 0.48 (0.26; 0.6) | - | ns |
| 1 | 0.48 (0.14; 0.6) | - | ||
| C18:3 n3 (Area %) | 0 | 0.52 (0.43; 0.71) | - | ns |
| 1 | 0.52 (0.46; 0.68) | - | ||
| C20:3 n6 (Area %) | 0 | 1.96 (1.46; 2.33) | - | ns |
| 1 | 1.89 (1.02; 2.3) | - | ||
| C20:4 n6 (Area %) | 0 | - | 6.39 ± 1.47 | ns |
| 1 | - | 6.12 ± 1.8 | ||
| C20:5 n3 (Area %) | 0 | 0.67 (0.4; 0.75) | - | ns |
| 1 | 0.66 (0.46; 0.81) | - | ||
| C22:6 (Area %) | 0 | - | 1.32 ± 0.54 | ns |
| 1 | - | 1.13 ± 0.62 | ||
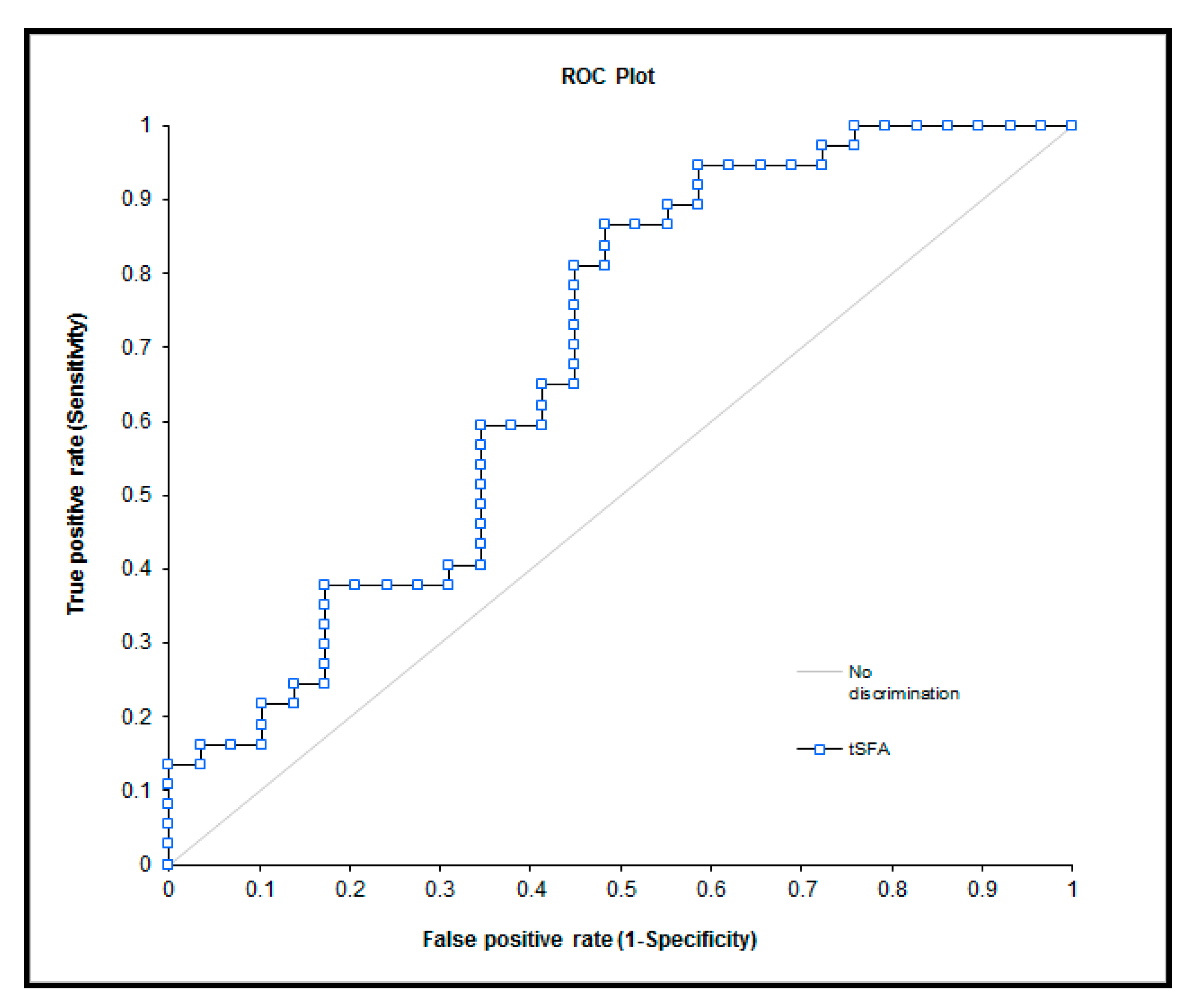
| tSFA | 0 | 32.61 (31.32; 36.26) | - | 0.01 |
| 1 | 34.21 (32.86; 39.39) | - | ||
| tMFA | 0 | - | 27.07 ± 3.3 | ns |
| 1 | - | 27.14 ± 3.2 | ||
| n6 | 0 | - | 35.64± 4.22 | ns |
| 1 | - | 34.02 ± 4.43 | ||
| n3 | 0 | 2.43 (1.97; 3.13) | - | ns |
| 1 | 2.35 (1.62; 3.12) | - | ||
| AA/EPA | 0 | 9.99 (7.72; 14.25) | - | ns |
| 1 | 9.6 (6.9; 11.96) | - | ||
| tPUFA | 0 | - | 38.4 ± 4.6 | ns |
| - | 36.4 ± 4.9 |
| Analyte | CIPN | Mean 0 ± SD | Mean 1 ± SD | Mean 2 ± SD | p Value |
|---|---|---|---|---|---|
| Vitamin B1 (ng/mL) | 0 | 15.12 ± 11.8 | 14.43 ± 8.1 | 11.48 ± 5.6 | ns |
| 1 | 11.5 ± 6.6 | 11.2 ± 5.4 | 9.3 ± 4 | ns | |
| Vitamin B6 (ng/mL) | 0 | 10.93 ± 9.7 | 10.08 ± 5.06 | 8.77 ± 5.3 | ns |
| 1 | 11.85 ± 9.7 | 9.64 ± 6.9 | 12.74 ± 9.9 | ns | |
| Vitamin D (nmol/L) | 0 | 38.08 ± 15.6 | 37.44 ± 19.9 | 42.33 ± 9.6 | <0.05 * |
| 1 | 26.94 ± 8.5 | 24.28 ± 8.9 | 31.4 ± 10.4 | <0.001 *,# | |
| C12:0 (Area %) | 0 | 0.28 ± 0.18 | 0.26 ± 0.14 | 0.41 ± 0.23 | <0.05 *,# |
| 1 | 0.36 ± 0.23 | 0.38 ± 0.2 | 0.34 ± 0.16 | ns | |
| C14:0 (Area %) | 0 | 1.53 ± 0.6 | 1.95 ± 0.9 | 2.5 ± 0.8 | <0.05 * |
| 1 | 1.94 ± 0.9 | 2.32 ± 0.9 | 2.1 ± 0.85 | <0.05 *,# | |
| C16:0 (Area %) | 0 | 25.23 ± 2.7 | 26.27 ± 3.6 | 29.44 ± 2.9 | <0.05 #,** |
| 1 | 26.49 ± 3.41 | 28.78 ± 3.6 | 29.45 ± 4.1 | <0.001 *,#,** | |
| C16:1 (Area %) | 0 | 2.42 ± 0.7 | 2.8 ± 0.9 | 2.89 ± 0.8 | ns |
| 1 | 2.96 ± 1.1 | 3.2 ± 0.84 | 2.96 ± 1.1 | ns | |
| C18:0 (Area %) | 0 | 7.03 ± 0.65 | 7 ± 0.72 | 6.76 ± 0.73 | ns |
| 1 | 7.24 ± 0.7 | 6.95 ± 0.65 | 7.1 ± 0.16 | <0.001 *,** | |
| C18:1 (Area %) | 0 | 24.65 ± 3 | 23.7 ± 3.4 | 23.78 ± 2.5 | ns |
| 1 | 24.18 ± 2.6 | 24.34 ± 2.7 | 23.22 ± 2.6 | ns | |
| C18:2 n6 (Area %) | 0 | 27.36 ± 3.9 | 27.11 ± 3.4 | 26.2 ± 3.76 | <0.05 *,# |
| 1 | 26.19 ± 3.7 | 25.2 ± 4 | 26.44 ± 4.1 | ns | |
| C18:3 n6 (Area %) | 0 | 0.46 ± 0.22 | 0.4 ± 0.25 | 0.17 ± 0.11 | <0.05 * |
| 1 | 0.42 ± 0.27 | 0.27 ± 0.23 | 0.23 ± 0.2 | <0.001 *,#,** | |
| C18:3 n3 (Area %) | 0 | 0.82 ± 0.65 | 1.02 ± 0.76 | 0.82 ± 0.62 | ns |
| 1 | 0.57 ± 0.2 | 0.54 ± 0.18 | 0.55 ± 0.2 | ns | |
| C20:3 n6 (Area %) | 0 | 1.89 ± 0.54 | 1.62 ± 0.56 | 1.14 ± 0.34 | <0.05 * |
| 1 | 1.72 ± 0.7 | 1.41 ± 0.63 | 1.24 ± 0.56 | <0.001 *,#,** | |
| C20:4 n6 (Area %) | 0 | 6.39 ± 1.47 | 5.89 ± 1.89 | 4.59 ± 1.3 | <0.05 * |
| 1 | 6.12 ± 1.8 | 5.22 ± 1.7 | 4.95 ± 1.5 | <0.001 *,#,** | |
| C20:5 n3 (Area %) | 0 | 0.62 ± 0.3 | 0.86 ± 0.56 | 0.74 ± 0.65 | ns |
| 1 | 0.68 ± 0.3 | 0.6 ± 0.28 | 0.71 ± 0.4 | ns | |
| C22:6 (Area %) | 0 | 1.32 ± 0.54 | 1.11 ± 0.6 | 0.56 ± 0.3 | <0.001 *,# |
| 1 | 1.13 ± 0.6 | 0.78 ± 0.6 | 0.72 ± 0.6 | <0.001 *,#,** | |
| tSFA | 0 | 34.1 ± 3.5 | 35.5 ± 4.15 | 39.11 ± 3.33 | <0.05 *,# |
| 1 | 36 ± 4.1 | 38.44 ± 4.4 | 38.98 ± 4.5 | <0.001 *,#,** | |
| tMFA | 0 | 27.1 ± 3.3 | 26.52 ± 3.7 | 26.67 ± 2.64 | ns |
| 1 | 27.14 ± 3.2 | 27.53 ± 3.1 | 26.2 ± 3 | ns | |
| n6 | 0 | 35.64 ± 4.2 | 34.61 ± 4.1 | 31.93 ± 3.62 | <0.05 *,# |
| 1 | 34.02 ± 4.42 | 31.83 ± 4.7 | 32.63 ± 4.9 | <0.001 *,** | |
| n3 | 0 | 2.76 ± 1.45 | 2.99 ± 2.23 | 2.12 ± 1.84 | <0.05 * |
| 1 | 2.38 ± 0.9 | 1.93 ± 0.98 | 1.97 ± 1.1 | <0.001 *,#,** | |
| AA/EPA | 0 | 13.1 ± 8.5 | 8.15 ± 2.72 | 8.1 ± 3.56 | ns |
| 1 | 9.92 ± 3.24 | 9.48 ± 3.15 | 8.44 ± 3.82 | <0.05 * | |
| tPUFA | 0 | 38.4 ± 4.64 | 37.6 ± 5.5 | 34.05 ± 4 | <0.001 *,# |
| 1 | 36.4 ± 4.9 | 33.75 ± 5 | 34.6 ± 5.4 | <0.001 *,#,** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grim, J.; Ticha, A.; Hyspler, R.; Valis, M.; Zadak, Z. Selected Risk Nutritional Factors for Chemotherapy-Induced Polyneuropathy. Nutrients 2017, 9, 535. https://doi.org/10.3390/nu9060535
Grim J, Ticha A, Hyspler R, Valis M, Zadak Z. Selected Risk Nutritional Factors for Chemotherapy-Induced Polyneuropathy. Nutrients. 2017; 9(6):535. https://doi.org/10.3390/nu9060535
Chicago/Turabian StyleGrim, Jiri, Alena Ticha, Radomir Hyspler, Martin Valis, and Zdenek Zadak. 2017. "Selected Risk Nutritional Factors for Chemotherapy-Induced Polyneuropathy" Nutrients 9, no. 6: 535. https://doi.org/10.3390/nu9060535




