Associations between Diet and Toenail Arsenic Concentration among Pregnant Women in Bangladesh: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Dietary Assessment
2.3. Arsenic Exposure Assessment
2.4. Statistical Analysis
3. Results
3.1. Comparison between Low and High Water As Exposure
3.2. Crude versus Adjusted Model
3.3. Comparison of Dietary Assessments 1 and 2
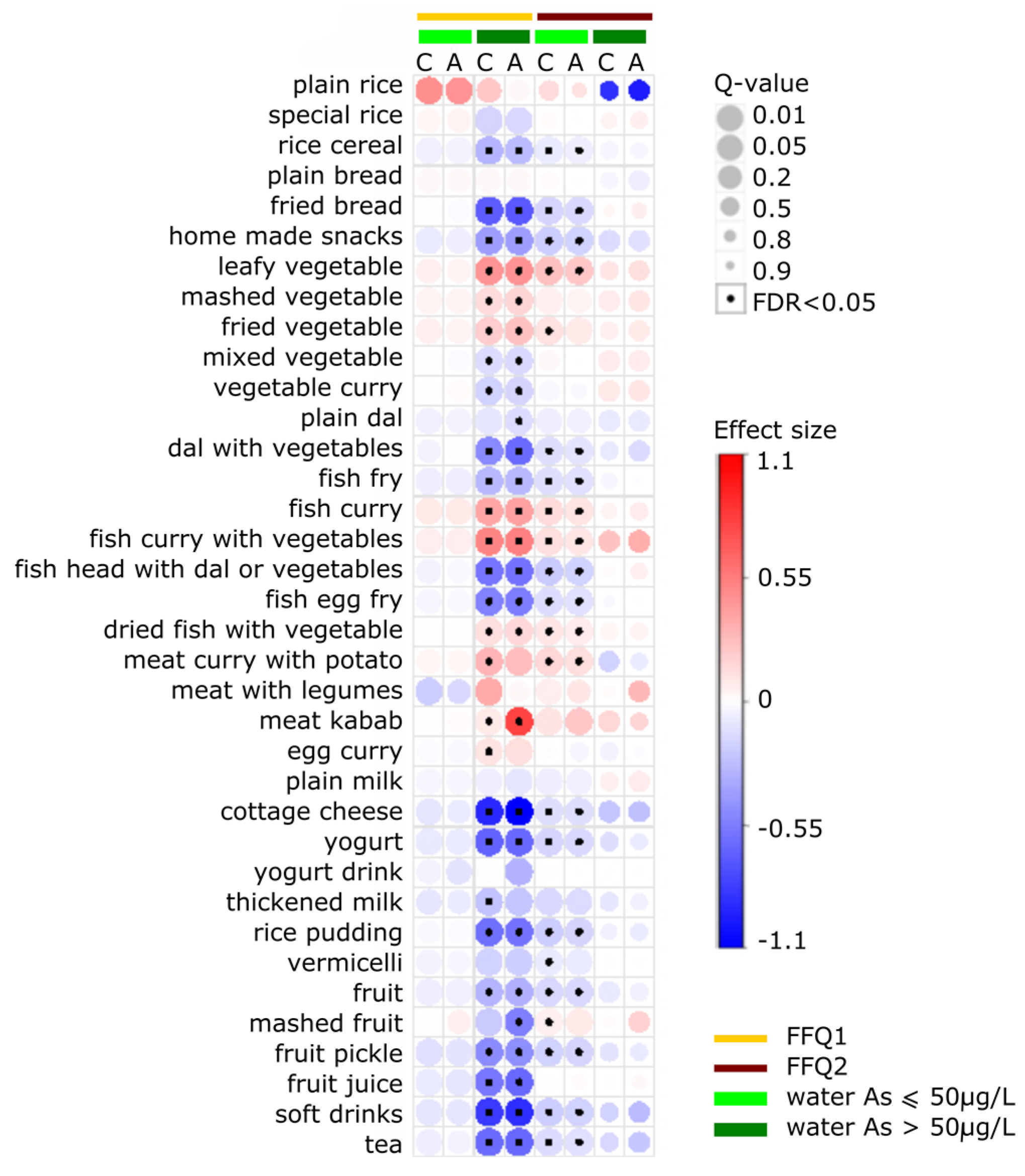
3.4. Food Items Positively Associated with ln-Transformed Toenail As
3.5. Food Items Negatively Associated with ln-Transformed Toenail As
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO Exposure to Arsenic: A Major Public Health Concern. Available online: http://www.who.int/ipcs/assessment/public_health/arsenic/en/ (accessed on 15 April 2015).
- Agency for Toxic Substances and Disease Registry (ATSDR). Agency for Toxic Substances and Disease Registry: Arsenic Toxicity: Environmental Alert; ATSDR: Atlanta, GA, USA, 2000. [Google Scholar]
- Tseng, C.-H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol. 2009, 235, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Rahman, M.M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, J.M.; Mayer, A.B.; Lauren, J.G.; Hassan, N. Food chain aspects of arsenic contamination in Bangladesh: Effects on quality and productivity of rice. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2003, 38, 61–69. [Google Scholar] [CrossRef]
- MacIntosh, D.L.; Spengler, J.D.; Ozkaynak, H.; Tsai, L.; Ryan, P.B. Dietary exposures to selected metals and pesticides. Environ. Health Perspect. 1996, 104, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, I.; Gómez, M.M.; Fodor, P.; Palacios, M.A.; Cámara, C. Distribution and biotransformation of arsenic species in chicken cardiac and muscle tissues. Biol. Trace Elem. Res. 2004, 99, 129–143. [Google Scholar] [CrossRef]
- Lasky, T.; Sun, W.; Kadry, A.; Hoffman, M.K. Mean total arsenic concentrations in chicken 1989–2000 and estimated exposures for consumers of chicken. Environ. Health Perspect. 2004, 112, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Borak, J.; Hosgood, H.D. Seafood arsenic: Implications for human risk assessment. Regul. Toxicol. Pharmacol. 2007, 47, 204–212. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar]
- Kile, M.L.; Houseman, E.A.; Breton, C.V.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Christiani, D.C. Association between total ingested arsenic and toenail arsenic concentrations. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2007, 42, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Kile, M.L.; Houseman, E.A.; Breton, C.V.; Smith, T.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Christiani, D.C. Dietary arsenic exposure in bangladesh. Environ. Health Perspect. 2007, 115, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Watanabe, C.; Inaoka, T.; Sekiyama, M.; Sudo, N.; Bokul, M.H.; Ohtsuka, R. Arsenic in cooked rice in Bangladesh. Lancet 2002, 360, 1839–1840. [Google Scholar] [CrossRef]
- Alam, M.G.M.; Snow, E.T.; Tanaka, A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total Environ. 2003, 308, 83–96. [Google Scholar] [CrossRef]
- Roychowdhury, T.; Tokunaga, H.; Ando, M. Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci. Total Environ. 2003, 308, 15–35. [Google Scholar] [CrossRef]
- Signes, A.; Mitra, K.; Burló, F.; Carbonell-Barrachina, A.A. Effect of cooking method and rice type on arsenic concentration in cooked rice and the estimation of arsenic dietary intake in a rural village in West Bengal, India. Food Addit. Contam. A 2008, 25, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.J.; Conklin, S.D.; Todorov, T.I.; Kasko, S.M. Cooking rice in excess water reduces both arsenic and enriched vitamins in the cooked grain. Food Addit. Contam. A 2016, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.; Jiujin, X.; Gomes Farias, J.; Meharg, A.A.; Meharg, A.; Williams, P.; Adamako, E.; Lawgali, Y.; Deacon, C.; Villada, A.; et al. Rethinking Rice Preparation for Highly Efficient Removal of Inorganic Arsenic Using Percolating Cooking Water. PLoS ONE 2015, 10, e0131608. [Google Scholar] [CrossRef] [PubMed]
- Cascio, C.; Raab, A.; Jenkins, R.O.; Feldmann, J.; Meharg, A.A.; Haris, P.I. The impact of a rice based diet on urinary arsenic. J. Environ. Monit. 2011, 13, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Diamond, D.; Cottingham, K.L.; Gruber, J.F.; Punshon, T.; Sayarath, V.; Gandolfi, A.J.; Baker, E.R.; Jackson, B.P.; Folt, C.L.; Karagas, M.R. Rice consumption contributes to arsenic exposure in US women. Proc. Natl. Acad. Sci. USA 2011, 108, 20656–20660. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, J.; Nguyen, A. Rice consumption and urinary concentrations of arsenic in US adults. Int. J. Environ. Health Res. 2014, 24, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H.; Anwar, M.; Horai, S.; Ando, T.; Nakano, A.; Wakamiya, J.; Koriyama, C.; Nakagawa, M.; Yamada, K.; Akiba, S. Toenail arsenic levels among residents in Amami-Oshima Island, Japan. Environ. Sci. 2006, 13, 149–160. [Google Scholar] [PubMed]
- Dabeka, R.W.; McKenzie, A.D.; Lacroix, G.M.; Cleroux, C.; Bowe, S.; Graham, R.A.; Conacher, H.B.; Verdier, P. Survey of arsenic in total diet food composites and estimation of the dietary intake of arsenic by Canadian adults and children. J. AOAC Int. 1993, 76, 14–25. [Google Scholar] [PubMed]
- MacIntosh, D.L.; Williams, P.L.; Hunter, D.J.; Sampson, L.A.; Morris, S.C.; Willett, W.C.; Rimm, E.B. Evaluation of a food frequency questionnaire-food composition approach for estimating dietary intake of inorganic arsenic and methylmercury. Cancer Epidemiol. Biomark. Prev. 1997, 6, 1043–1050. [Google Scholar] [PubMed]
- Schoof, R.A.; Yost, L.J.; Eickhoff, J.; Crecelius, E.A.; Cragin, D.W.; Meacher, D.M.; Menzel, D.B. A market basket survey of inorganic arsenic in food. Food Chem. Toxicol. 1999, 37, 839–846. [Google Scholar] [CrossRef]
- Meharg, A.A.; Deacon, C.; Campbell, R.C.J.; Carey, A.-M.; Williams, P.N.; Feldmann, J.; Raab, A. Inorganic arsenic levels in rice milk exceed EU and US drinking water standards. J. Environ. Monit. 2008, 10, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Sun, G.; Williams, P.N.; Adomako, E.; Deacon, C.; Zhu, Y.-G.; Feldmann, J.; Raab, A. Inorganic arsenic levels in baby rice are of concern. Environ. Pollut. 2008, 152, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.S.-H.; Michael Bolger, P. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991–December 1996. Food Addit. Contam. 1999, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, B.R.; Caldwell, K.L.; Jones, R.L.; Blount, B.C.; Pan, Y.; Ward, C.; Mortensen, M.E.; Chen, C.; Hsueh, Y.; Lai, M.; et al. Dietary Sources of Methylated Arsenic Species in Urine of the United States Population, NHANES 2003–2010. PLoS ONE 2014, 9, e108098. [Google Scholar] [CrossRef] [PubMed]
- Das, H.K.; Mitra, A.K.; Sengupta, P.K.; Hossain, A.; Islam, F.; Rabbani, G.H. Arsenic concentrations in rice, vegetables, and fish in Bangladesh: A preliminary study. Environ. Int. 2004, 30, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, K.L.; Karimi, R.; Gruber, J.F.; Zens, M.S.; Sayarath, V.; Folt, C.L.; Punshon, T.; Morris, J.S.; Karagas, M.R. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. Nutr. J. 2013, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shi, J.; Jiang, G.; Jing, C. Arsenic Levels and Speciation from Ingestion Exposures to Biomarkers in Shanxi, China: Implications for Human Health. Environ. Sci. Technol. 2013, 47, 5419–5424. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Vedel-Macrander, G.C.; Zaworotko, M.J.; Tatum, F.M.; Meeks, R.G. Distribution, metabolism, and fetal uptake of pentavalent arsenic in pregnant mice following oral or intraperitoneal administration. Teratology 1987, 35, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Vedel, G.C.; Zaworotko, M.J.; Tatum, F.M.; Meeks, R.G. Uptake, distribution, and metabolism of trivalent arsenic in the pregnant mouse. J. Toxicol. Environ. Health 1988, 25, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, B.J.; Bennett, G.D.; Calvin, J.A.; Finnell, R.H. Arsenic-induced neural tube defects in mice: Alterations in cell cycle gene expression. Reprod. Toxicol. 1996, 10, 447–454. [Google Scholar] [CrossRef]
- Quansah, R.; Armah, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfo, K.; Cobbina, S.J.; Nketiah-Amponsah, E.; Namujju, P.B.; Obiri, S.; et al. Association of Arsenic with Adverse Pregnancy Outcomes-Infant Mortality: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Piao, F.; Zhang, X.; Li, X.; Li, Q.; Xu, L.; Kitamura, F.; Yokoyama, K. Prenatal exposure to arsenic and its effects on fetal development in the general population of Dalian. Biol. Trace Elem. Res. 2012, 149, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yokoyama, K.; Tian, Y.; Piao, F.-Y.; Kitamura, F.; Kida, H.; Wang, P. Decrease in birth weight and gestational age by arsenic among the newborn in Shanghai, China. Nihon Koshu Eisei Zasshi 2011, 58, 89–95. [Google Scholar] [PubMed]
- Smith, A.H.; Hopenhayn-Rich, C.; Bates, M.N.; Goeden, H.M.; Hertz-Picciotto, I.; Duggan, H.M.; Wood, R.; Kosnett, M.J.; Smith, M.T. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992, 97, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Lönnerdal, B.; Stoltzfus, R.J. Interactions between nutrition and environmental exposures: Effects on health outcomes in women and children. J. Nutr. 2007, 137, 2794–2797. [Google Scholar] [PubMed]
- Huyck, K.L.; Kile, M.L.; Mahiuddin, G.; Quamruzzaman, Q.; Rahman, M.; Breton, C.V.; Dobson, C.B.; Frelich, J.; Hoffman, E.; Yousuf, J.; et al. Maternal Arsenic Exposure Associated With Low Birth Weight in Bangladesh. J. Occup. Environ. Med. 2007, 49, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Kile, M.L.; Rodrigues, E.G.; Mazumdar, M.; Dobson, C.B.; Diao, N.; Golam, M.; Quamruzzaman, Q.; Rahman, M.; Christiani, D.C. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ. Health 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Kile, M.; Dobson, C.; Amarasiriwardena, C.; Quamruzzaman, Q.; Rahman, M.; Golam, M.; Christiani, D.C. Maternal–infant biomarkers of prenatal exposure to arsenic and manganese. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Kile, M.L.; Cardenas, A.; Rodrigues, E.; Mazumdar, M.; Dobson, C.; Golam, M.; Quamruzzaman, Q.; Rahman, M.; Christiani, D.C. Estimating Effects of Arsenic Exposure During Pregnancy on Perinatal Outcomes in a Bangladeshi Cohort. Epidemiology 2016, 27, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-I.; Bromage, S.; Mostofa, M.; Allen, J.; Oken, E.; Kile, M.; Christiani, D. Validation of a Dish-Based Semiquantitative Food Questionnaire in Rural Bangladesh. Nutrients 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Willett, W.C. Self-administered semiquantitative food frequency questionnaires: Patterns, predictors, and interpretation of omitted items. Epidemiology 2009, 20, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Rahim, A.T.; Banu, M.C.P.; Bari, L.; Tukun, B.; Mannan, M.; Bhattacharjee, L.; Stadlmayr, B. Food Composition Table for Bangladesh; University of Dhaka: Dhaka, Bangladesh, 2013. [Google Scholar]
- Karagas, M.R.; Morris, J.S.; Weiss, J.E.; Spate, V.; Baskett, C.; Greenberg, E.R. Toenail samples as an indicator of drinking water arsenic exposure. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 849–852. [Google Scholar] [PubMed]
- Button, M.; Jenkin, G.R.T.; Harrington, C.F.; Watts, M.J. Human toenails as a biomarker of exposure to elevated environmental arsenic. J. Environ. Monit. 2009, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Amarasiriwardena, C.J.; Christiani, D.C. Determination of total arsenic concentrations in nails by inductively coupled plasma mass spectrometry. Biol. Trace Elem. Res. 1999, 67, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Catelan, D.; Biggeri, A. Multiple testing in disease mapping and descriptive epidemiology. Geospat. Health 2010, 4, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Dabney, A.; Robinson, D. Qvalue: Q-Value Estimation for False Discovery Rate Control. R Packag. Version 2.6.0. 2015. Available online: http://github.com/jdstorey/qvalue/ (accessed on 1 October 2015).
- Haarman, B.C.M.; Riemersma-Van der Lek, R.F.; Nolen, W.A.; Mendes, R.; Drexhage, H.A.; Burger, H. Feature-expression heat maps—A new visual method to explore complex associations between two variable sets. J. Biomed. Inform. 2015, 53, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Sturup, S.; Chen, C.; Folt, C.; Karagas, M.R. Toenail mercury and dietary fish consumption. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-L.B.; Amarasiriwardena, C.J.; Christiani, D.C. Determination of total arsenic concentrations in nails by inductively coupled plasma mass spectrometry. Biol. Trace Elem. Res. 1999, 67, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn, C.; Huang, B.; Christian, J.; Peralta, C.; Ferreccio, C.; Atallah, R.; Kalman, D. Profile of urinary arsenic metabolites during pregnancy. Environ. Health Perspect. 2003, 111, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.-L.; Ekström, E.-C.; Nermell, B.; Rahman, M.; Lönnerdal, B.; Persson, L.-Å.; Vahter, M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res. 2008, 106, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Nermell, B.; Kippler, M.; Grandér, M.; Li, L.; Ekström, E.-C.; Rahman, A.; Lönnerdal, B.; Hoque, A.M.W.; Vahter, M. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod. Toxicol. 2011, 31, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Engström, K.; Bottai, M.; Hoque, W.A.M.; Raqib, R.; Broberg, K.; Vahter, M. Pregnancy and the methyltransferase genotype independently influence the arsenic methylation phenotype. Pharmacogenet. Genom. 2012, 22, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Bailey, K.A.; Rubio-Andrade, M.; Olshan, A.F.; Smeester, L.; Drobná, Z.; Herring, A.H.; Stýblo, M.; García-Vargas, G.G.; Fry, R.C. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ. Health Perspect. 2015, 123, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; Gamble, M.V.; Chen, Y.; Graziano, J.H.; Slavkovich, V.; Parvez, F.; Baron, J.A.; Howe, G.R.; Ahsan, H. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am. J. Clin. Nutr. 2007, 85, 1367–1374. [Google Scholar] [PubMed]
- Melkonian, S.; Argos, M.; Chen, Y.; Parvez, F.; Pierce, B.; Ahmed, A.; Islam, T.; Ahsan, H. Intakes of several nutrients are associated with incidence of arsenic-related keratotic skin lesions in Bangladesh. J. Nutr. 2012, 142, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Van Geen, A.; Zheng, Y.; Cheng, Z.; He, Y.; Dhar, R.K.; Garnier, J.M.; Rose, J.; Seddique, A.; Hoque, M.A.; Ahmed, K.M. Impact of irrigating rice paddies with groundwater containing arsenic in Bangladesh. Sci. Total Environ. 2006, 367, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Islam, M.R.; Adomako, E.E.; Raab, A.; Hossain, S.A.; Zhu, Y.G.; Feldmann, J.; Meharg, A.A. Increase in Rice Grain Arsenic for Regions of Bangladesh Irrigating Paddies with Elevated Arsenic in Groundwaters. Environ. Sci. Technol. 2006, 40, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O.; Lin, T.-S. Trace metals in wild rice sold in the United States. Sci. Total Environ. 1995, 172, 223–228. [Google Scholar] [CrossRef]
- Williams, P.N.; Price, A.H.; Raab, A.; Hossain, S.A.; Feldmann, J.; Meharg, A.A. Variation in Arsenic Speciation and Concentration in Paddy Rice Related to Dietary Exposure. Environ. Sci. Technol. 2005, 39, 5531–5540. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Raab, A.; Feldmann, J.; Meharg, A.A. Market Basket Survey Shows Elevated Levels of As in South Central USA Processed Rice Compared to California: Consequences for Human Dietary Exposure. Environ. Sci. Technol. 2007, 41, 2178–2183. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Mitra, S.; Chung, J.; Guha Mazumder, D.N.; Ghosh, N.; Kalman, D.; von Ehrenstein, O.S.; Steinmaus, C.; Liaw, J.; Smith, A.H. Creatinine, Diet, Micronutrients, and Arsenic Methylation in West Bengal, India. Environ. Health Perspect. 2011, 119, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Vélez, D.; Barberá, R.; Farré, R.; Montoro, R. Bioavailability of Inorganic Arsenic in Cooked Rice: Practical Aspects for Human Health Risk Assessments. J. Agric. Food Chem. 2005, 53, 8829–8833. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zheng, Y. Assessment of in vivo bioaccessibility of arsenic in dietary rice by a mass balance approach. Sci. Total Environ. 2010, 408, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Mackenzie, T.A.; Cottingham, K.L.; Gilbert-Diamond, D.; Punshon, T.; Karagas, M.R. Rice Consumption and Urinary Arsenic Concentrations in USA Children. Environ. Health Perspect. 2012, 120, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.-S.; Bundschuh, J.; Bhattacharya, P. Arsenic in Geosphere and Human Diseases, (Arsenic 2010). In Proceedings of the 3rd International Congress on Arsenic in the Environment (As-2010), Tainan, Taiwan, 17–21 May 2010; CRC Press: London, UK; New York, NY, USA, 2010. [Google Scholar]
- Rehman, Z.U.; Khan, S.; Qin, K.; Brusseau, M.L.; Shah, M.T.; Din, I. Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Sci. Total Environ. 2016, 550, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zartarian, V.; Wang, S.-W.; Liu, S.V.; Georgopoulos, P. Probabilistic Modeling of Dietary Arsenic Exposure and Dose and Evaluation with 2003–2004 NHANES Data. Environ. Health Perspect. 2009, 118, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Awata, H.; Linder, S.; Mitchell, L.E.; Delclos, G.L. Association of Dietary Intake and Biomarker Levels of Arsenic, Cadmium, Lead, and Mercury among Asian Populations in the USA: NHANES 2011–2012. Environ. Health Perspect. 2017, 125, 314–323. [Google Scholar] [PubMed]
- Gagnon, F.; Lampron-Goulet, E.; Normandin, L.; Langlois, M.-F. Measurements of Arsenic in the Urine and Nails of Individuals Exposed to Low Concentrations of Arsenic in Drinking Water From Private Wells in a Rural Region of Québec, Canada. J. Environ. Health 2016, 78, 76–83. [Google Scholar] [PubMed]
- Molin, M.; Ulven, S.M.; Meltzer, H.M.; Alexander, J. Arsenic in the human food chain, biotransformation and toxicology—Review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 2015, 31, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Slotnick, M.J.; Meliker, J.R.; Kannan, S.; Nriagu, J.O. Effects of nutritional measures on toenail arsenic concentration as a biomarker of arsenic exposure. Biomarkers 2008, 13, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.F.; Karagas, M.R.; Gilbert-Diamond, D.; Bagley, P.J.; Zens, M.S.; Sayarath, V.; Punshon, T.; Morris, J.S.; Cottingham, K.L. Associations between toenail arsenic concentration and dietary factors in a New Hampshire population. Nutr. J. 2012, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Alava, P.; Du Laing, G.; Tack, F.; De Ryck, T.; Van De Wiele, T. Westernized diets lower arsenic gastrointestinal bioaccessibility but increase microbial arsenic speciation changes in the colon. Chemosphere 2015, 119, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahman, F.; Sansom, L.; Naidu, R.; Schmidt, O. Arsenic interactions with lipid particles containing iron. Environ. Geochem. Health 2009, 31, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Willet, W. Food Frequency Methods. In Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2013; pp. 71–72. [Google Scholar]
- World Bank. Bangladesh—Household Income and Expenditure Survey: Key Findings and Results 2010; World Bank: Washington, DC, USA, 2011. [Google Scholar]
- Willett, W. Nutritional Epidemiology: CH 13 Issues in Analysis and Presentation of Dietary Data; Oxford Scholarship: Oxford, UK, 2012. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1998. [Google Scholar]
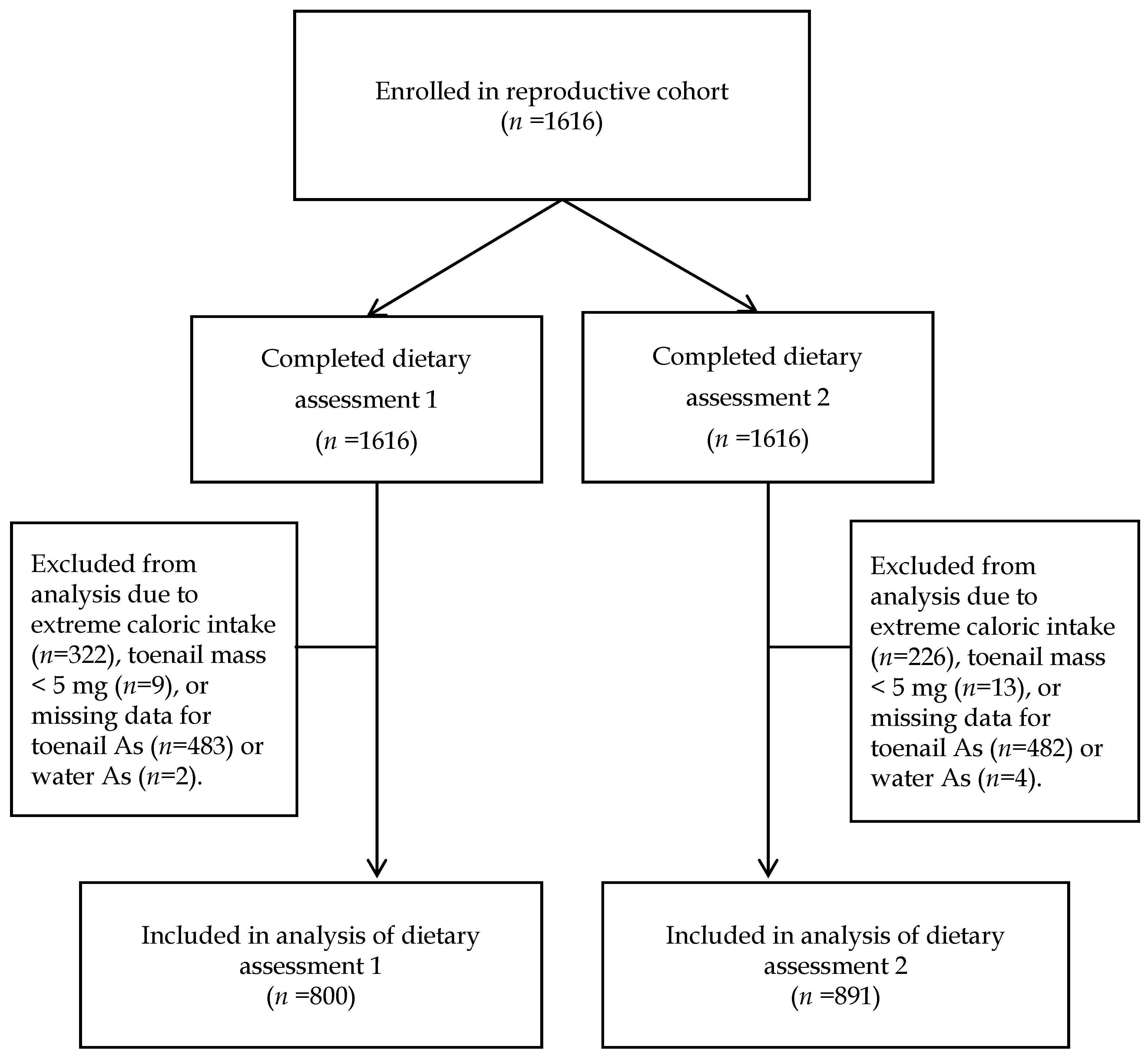
| Group | 1st Dietary Assessment (FFQ1) | 2nd Dietary Assessment (FFQ2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 800) | Water [As] ≤ 50 μg/L (n = 629) | Water [As] > 50 μg/L (n = 171) | Total (n = 891) | Water [As] ≤ 50 μg/L (n = 683) | Water [As] > 50 μg/L (n = 208) | |||||||||
| Continuous Variables | Mean | SD | Mean | SD | Mean | SD | p-Value * | Mean | SD | Mean | SD | Mean | SD | p-Value * |
| Age (years) | 23.0 | 4.3 | 23.0 | 4.3 | 23.2 | 4.3 | 0.590 | 23.0 | 4.2 | 23.0 | 4.3 | 23.1 | 3.9 | 0.764 |
| Toenail [As] (μg/g) | 3.3 | 4.6 | 2.3 | 3.5 | 7.1 | 5.8 | <0.001 | 2.7 | 4.1 | 1.8 | 3.3 | 5.8 | 4.7 | <0.001 |
| Water [As] (μg/L) | 45.8 | 111.6 | 6.8 | 11.1 | 188.9 | 178.6 | <0.001 | 50.0 | 113.2 | 8.6 | 12.7 | 210.6 | 193.1 | <0.001 |
| Water intake (L/day) | 2.1 | 0.5 | 2.2 | 0.5 | 2.0 | 0.4 | <0.001 | 2.2 | 0.4 | 2.2 | 0.4 | 2.1 | 0.4 | 0.002 |
| Energy intake (kcal/day) | 2804.2 | 417.5 | 2787.6 | 416.7 | 2865.0 | 416.1 | 0.032 | 2806.2 | 396.1 | 2777.5 | 389.9 | 2900.7 | 402.4 | <0.001 |
| Dichotomous Variables | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Sex (female) | 800 | 100 | 629 | 100 | 171 | 100 | 1.000 | 891 | 100.0 | 683 | 100.0 | 208 | 100.0 | 1.000 |
| Betel nut | 6 | 0.8 | 4 | 0.6 | 2 | 1.2 | 0.614 | 8 | 0.9 | 6 | 0.9 | 2 | 1.0 | 0.591 |
| Chew tobacco | 4 | 0.5 | 2 | 0.3 | 2 | 1.2 | 0.202 | 6 | 0.7 | 4 | 0.6 | 2 | 1.0 | 0.427 |
| Environmental smoke | 339 | 42.4 | 251 | 39.9 | 88 | 51.5 | 0.010 | 362 | 40.6 | 257 | 37.6 | 105 | 50.5 | 0.001 |
| Smoker | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.000 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.000 |
| Categorical Variables | N | % | N | % | N | % | N | % | N | % | N | % | ||
| BMI | 0.209 | - | - | - | - | - | - | 0.016 | ||||||
| Underweight (<18.5) | 231 | 28.9 | 173 | 27.5 | 58 | 33.9 | 241 | 27.0 | 168 | 24.6 | 73 | 27.0 | ||
| Normal (18.5 ≤ BMI < 25) | 497 | 62.1 | 395 | 62.8 | 102 | 59.6 | 569 | 63.9 | 447 | 65.4 | 122 | 63.9 | ||
| Overweight (25 ≤ BMI < 30) | 65 | 8.1 | 56 | 8.9 | 9 | 5.3 | 71 | 8.0 | 59 | 8.6 | 12 | 8.0 | ||
| Obese (≥30) | 7 | 0.9 | 5 | 0.8 | 2 | 1.2 | 10 | 1.1 | 9 | 1.3 | 1 | 1.1 | ||
| Education | 0.378 | - | - | - | - | - | - | 0.499 | ||||||
| Illiterate | 12 | 1.5 | 10 | 1.6 | 2 | 1.2 | 10 | 1.1 | 8 | 1.2 | 2 | 1.0 | ||
| Able to write | 106 | 13.3 | 81 | 12.9 | 25 | 14.6 | 125 | 14.0 | 94 | 13.7 | 31 | 14.9 | ||
| Primary education | 271 | 33.9 | 218 | 34.7 | 53 | 31.0 | 292 | 32.8 | 236 | 34.6 | 56 | 26.9 | ||
| Secondary education | 385 | 48.1 | 298 | 47.4 | 87 | 50.9 | 431 | 48.4 | 319 | 46.7 | 112 | 53.9 | ||
| Higher secondary education | 24 | 3.0 | 21 | 3.3 | 3 | 1.8 | 27 | 3.0 | 21 | 3.1 | 6 | 2.9 | ||
| College/graduate | 1 | 0.1 | 0 | 0.0 | 1 | 0.6 | 4 | 0.4 | 3 | 0.4 | 1 | 0.5 | ||
| Post-graduate | 1 | 0.1 | 1 | 0.2 | 0 | 0.0 | 2 | 0.2 | 2 | 0.3 | 0 | 0.0 | ||
| Food Items | 1st Dietary Assessment | 2nd Dietary Assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water [As] ≤ 50 μg/L (n = 629) | Water [As] > 50 μg/L (n = 171) | Water [As] ≤ 50 μg/L (n = 683) | Water [As] > 50 μg/L (n = 208) | |||||||||
| Daily Intake ∞ | % Change Toenail As ⌘ | Daily Intake ∞ | % Change Toenail As ⌘ | Daily Intake ∞ | % Change Toenail As ⌘ | Daily Intake ∞ | % Change Toenail As ⌘ | |||||
| Mean | STD | Mean | STD | Mean | STD | Mean | STD | |||||
| Grain, Cereal, Bread | ||||||||||||
| Plain rice (Bhaat, Panta bhaat) | 1355.14 | 160.79 | 13.65 | 1396.38 | 151.84 | 0.79 | 1331.07 | 150.00 | 4.95 | 1369.2 | 107.1 | −3.22 |
| Special rice (Khichuri, Pulao, Biriyani) | 25.83 | 30.67 | 6.47 | 25.04 | 30.21 | −26.23 | 25.51 | 27.68 | −0.48 | 24.6 | 23.7 | 9.89 |
| Rice cereal (Chira, Muri, Khoi, Murki) | 68.60 | 56.93 | −16.40 | 29.12 | 42.02 | −62.00 * | 62.42 | 52.53 | −21.67 * | 14.5 | 20.3 | −8.43 |
| Plain bread (Atta ruti, Pau ruti) | 84.60 | 92.94 | 6.28 | 81.20 | 79.88 | 7.73 | 98.22 | 103.74 | −1.84 | 73.5 | 65.7 | −13.32 |
| Fried bread (Porota, Luchi) | 22.37 | 20.17 | −3.46 | 12.67 | 6.24 | −61.80 * | 33.52 | 25.52 | −27.46 * | 14.5 | 12.3 | 8.76 |
| Home made snacks (Pitha-puli) | 31.96 | 32.74 | −15.21 | 17.23 | 22.38 | −61.77 * | 32.06 | 25.40 | −37.83 * | 12.1 | 8.3 | −21.34 |
| Vegetable | ||||||||||||
| Leafy vegetable (Sak) | 56.03 | 41.31 | 6.29 | 80.86 | 41.32 | 85.95 * | 54.54 | 37.07 | 36.43 * | 91.5 | 35.7 | 9.35 |
| Mashed vegetable (Bhorta) | 24.87 | 18.92 | 13.05 | 29.70 | 21.10 | 59.42 * | 20.16 | 20.86 | 12.59 | 29.7 | 21.3 | 30.41 |
| Fried vegetable (Bhaji) | 50.82 | 32.08 | 15.33 | 66.77 | 34.89 | 97.20 * | 49.04 | 31.13 | 27.93 | 71.4 | 36.8 | 28.30 |
| Mixed vegetable (Labra) | 41.88 | 34.47 | −4.79 | 43.66 | 44.04 | −39.72 * | 35.00 | 33.09 | −0.10 | 38.7 | 41.6 | 31.97 |
| Vegetable Curry (Torkarir jhole) | 51.91 | 36.11 | 3.13 | 51.96 | 51.61 | −45.60 * | 47.47 | 32.28 | −4.94 | 39.3 | 44.7 | 38.48 |
| Legumes, Pulses, Seeds | ||||||||||||
| Plain dal | 67.82 | 38.63 | −12.83 | 58.72 | 42.30 | −39.28 * | 64.22 | 30.46 | −12.07 | 57.6 | 37.7 | −25.19 |
| Dal with vegetables | 10.77 | 5.00 | −0.11 | 9.99 | 3.94 | −49.19 * | 44.52 | 34.67 | −28.83 * | 7.8 | 9.8 | −12.57 |
| Fish, Poultry, Meat, Egg | ||||||||||||
| Fish Fry (Mach bhaji) | 194.79 | 128.82 | −18.61 | 101.51 | 101.42 | −54.10 * | 197.75 | 147.83 | −30.76 * | 56.3 | 65.7 | −1.26 |
| Fish curry (Mach er jhole) | 126.17 | 101.19 | 36.93 | 211.48 | 92.26 | 116.90 * | 119.67 | 98.76 | 47.27 * | 217.8 | 83.6 | 17.76 |
| Fish curry with vegetable | 156.39 | 121.88 | 21.21 | 271.76 | 93.54 | 189.10 * | 160.99 | 128.42 | 24.30 * | 301.9 | 61.3 | 26.18 |
| Fish head with dal or vegetables | 12.64 | 10.83 | −5.62 | 7.05 | 5.07 | −74.44 * | 10.98 | 11.12 | −28.62 * | 5.1 | 3.3 | 13.42 |
| Fish egg fry (Maccher dim bhaji) | 4.87 | 4.00 | −6.83 | 2.00 | 2.44 | −77.12 * | 8.05 | 8.25 | −35.29* | 1.3 | 1.7 | 0.26 |
| Dried fish with vegetable | 94.79 | 116.21 | −1.01 | 157.31 | 128.09 | 64.23 * | 102.32 | 129.05 | 38.37 * | 210.9 | 122.9 | 13.71 |
| Meat curry with potato | 32.60 | 34.24 | 9.51 | 36.34 | 19.05 | 20.64 | 24.65 | 20.37 | 41.15 * | 37.3 | 25.6 | −5.22 |
| Meat with legumes (Halim) | 12.28 | 4.06 | −13.98 | 12.18 | 3.69 | 2.98 | 16.64 | 13.17 | 32.98 | 23.7 | 15.2 | 102.25 |
| Meat kebab | 171.36 | 555.09 | 9.20 | 200.26 | 415.79 | 3321.24 * | 96.46 | 66.16 | 54.79 | 130.9 | 82.9 | 37.86 |
| Egg curry (Dim er jhole) | 98.57 | 84.90 | −7.24 | 134.87 | 107.91 | 43.85 | 96.42 | 71.96 | −8.45 | 139.9 | 103.1 | −3.31 |
| Milk | ||||||||||||
| Plain milk (Doodh) | 127.10 | 92.50 | −13.78 | 81.47 | 73.91 | −32.26 | 132.31 | 87.45 | −13.33 | 70.7 | 58.2 | 36.04 |
| Cottage cheese (chana) | 2.45 | 1.24 | −11.58 | 1.60 | 0.94 | −79.78 * | 3.88 | 3.28 | −32.93 * | 0.9 | 1.0 | −28.02 |
| Yogurt (Doi) | 5.20 | 4.50 | −13.87 | 2.88 | 2.66 | −71.94 * | 4.74 | 3.17 | −25.05 * | 1.7 | 1.0 | −9.96 |
| Yogurt drink (Ghole, Matha, Borhani) | 7.13 | 4.89 | −21.63 | 5.97 | 3.71 | −44.92 | NA | NA | NA | NA | NA | NA |
| Thickened milk (Khoa, kheer) | 11.83 | 7.83 | −10.20 | 10.08 | 5.99 | −27.50 | 11.40 | 4.86 | −18.26 | 9.8 | 7.5 | −15.66 |
| Rice pudding (Payesh) | 15.07 | 11.86 | −2.96 | 8.54 | 5.48 | −68.47 * | 14.85 | 15.51 | −32.05 * | 6.8 | 7.2 | −9.59 |
| Vermicelli (Semai) | 30.15 | 27.62 | −9.81 | 22.91 | 21.91 | −23.35 | 33.42 | 30.39 | −18.49 | 20.9 | 15.4 | 1.16 |
| Fruits | ||||||||||||
| Fruit | 78.85 | 62.33 | −17.87 | 32.39 | 39.69 | −58.55 * | 79.74 | 55.88 | −38.10 * | 37.3 | 109.8 | −13.78 |
| Mashed fruit (Bhorta) | 2.32 | 1.12 | 7.93 | 2.53 | 4.20 | −49.13 * | 5.34 | 9.08 | 41.70 | 9.6 | 10.1 | 77.70 |
| Fruit pickle (Aachar) | 1.67 | 3.15 | −24.05 | 1.26 | 1.79 | −78.20 * | 1.95 | 1.61 | −37.58 * | 0.7 | 0.6 | −15.64 |
| Beverages | ||||||||||||
| Fruit juice | 26.09 | 19.30 | −21.87 | 12.85 | 12.28 | −76.25 * | 4071.49 | 36,352.71 | 15.73 | 2343.3 | 18,866.8 | 21.76 |
| Soft drinks | 17.33 | 9.56 | −15.11 | 10.22 | 5.78 | −75.45 * | 16.42 | 10.75 | −28.95 * | 7.0 | 2.3 | −20.71 |
| Tea | 36.63 | 27.29 | −9.65 | 21.58 | 23.80 | −77.18 * | 40.64 | 31.81 | −26.73 * | 11.7 | 10.0 | −20.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-I.D.; Bromage, S.; Mostofa, M.G.; Allen, J.; Oken, E.; Kile, M.L.; Christiani, D.C. Associations between Diet and Toenail Arsenic Concentration among Pregnant Women in Bangladesh: A Prospective Study. Nutrients 2017, 9, 420. https://doi.org/10.3390/nu9040420
Lin P-ID, Bromage S, Mostofa MG, Allen J, Oken E, Kile ML, Christiani DC. Associations between Diet and Toenail Arsenic Concentration among Pregnant Women in Bangladesh: A Prospective Study. Nutrients. 2017; 9(4):420. https://doi.org/10.3390/nu9040420
Chicago/Turabian StyleLin, Pi-I. D., Sabri Bromage, Md. Golam Mostofa, Joseph Allen, Emily Oken, Molly L. Kile, and David C. Christiani. 2017. "Associations between Diet and Toenail Arsenic Concentration among Pregnant Women in Bangladesh: A Prospective Study" Nutrients 9, no. 4: 420. https://doi.org/10.3390/nu9040420






