Nutrient Status of Vitamin D among Chinese Children
Abstract
:1. Introduction
2. Participants and Methods
2.1. Study Population
2.2. 25-Hydroxyvitamin D Measurement
2.3. Assessment of Vitamin D Status
2.4. Statistical Analyses
3. Results
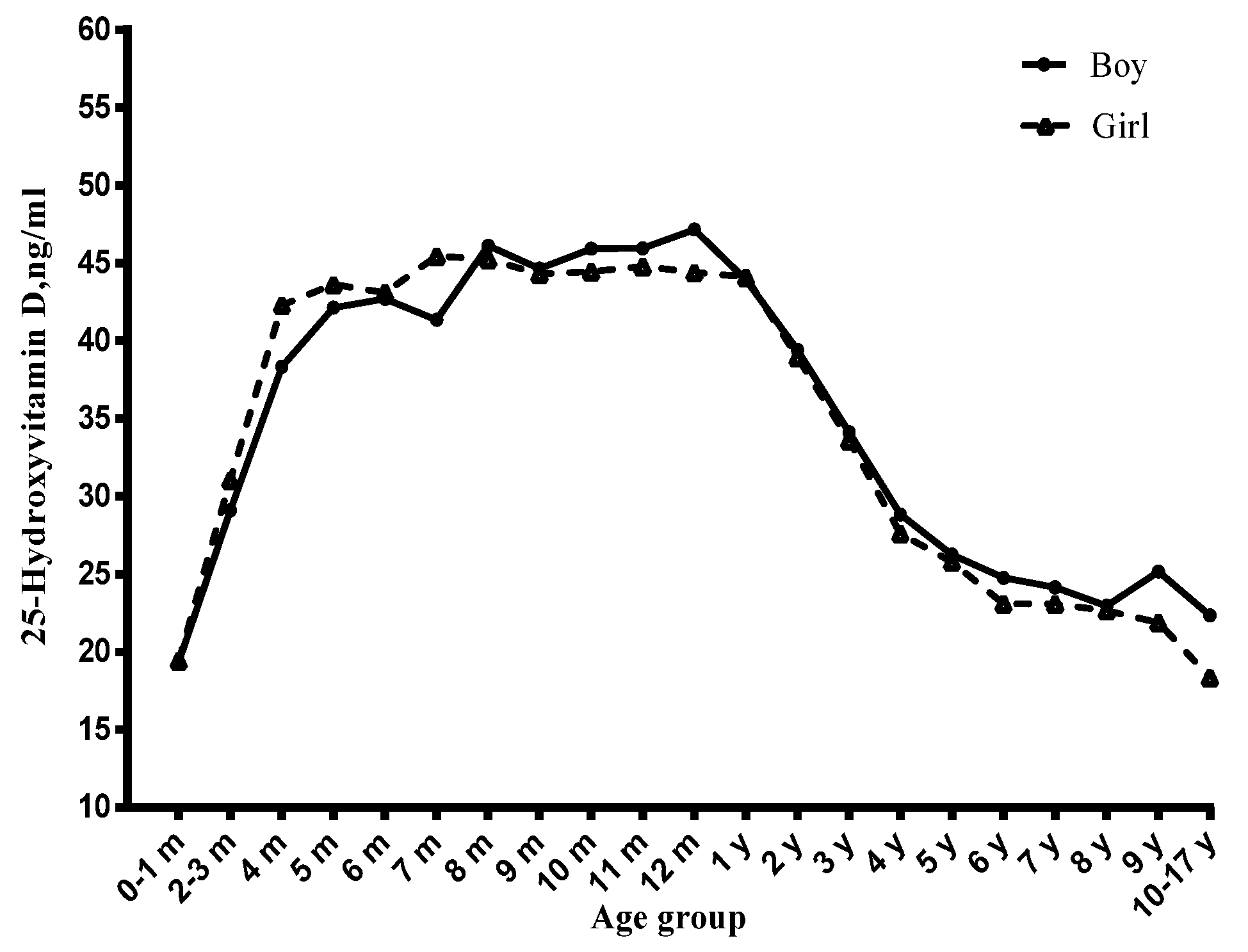
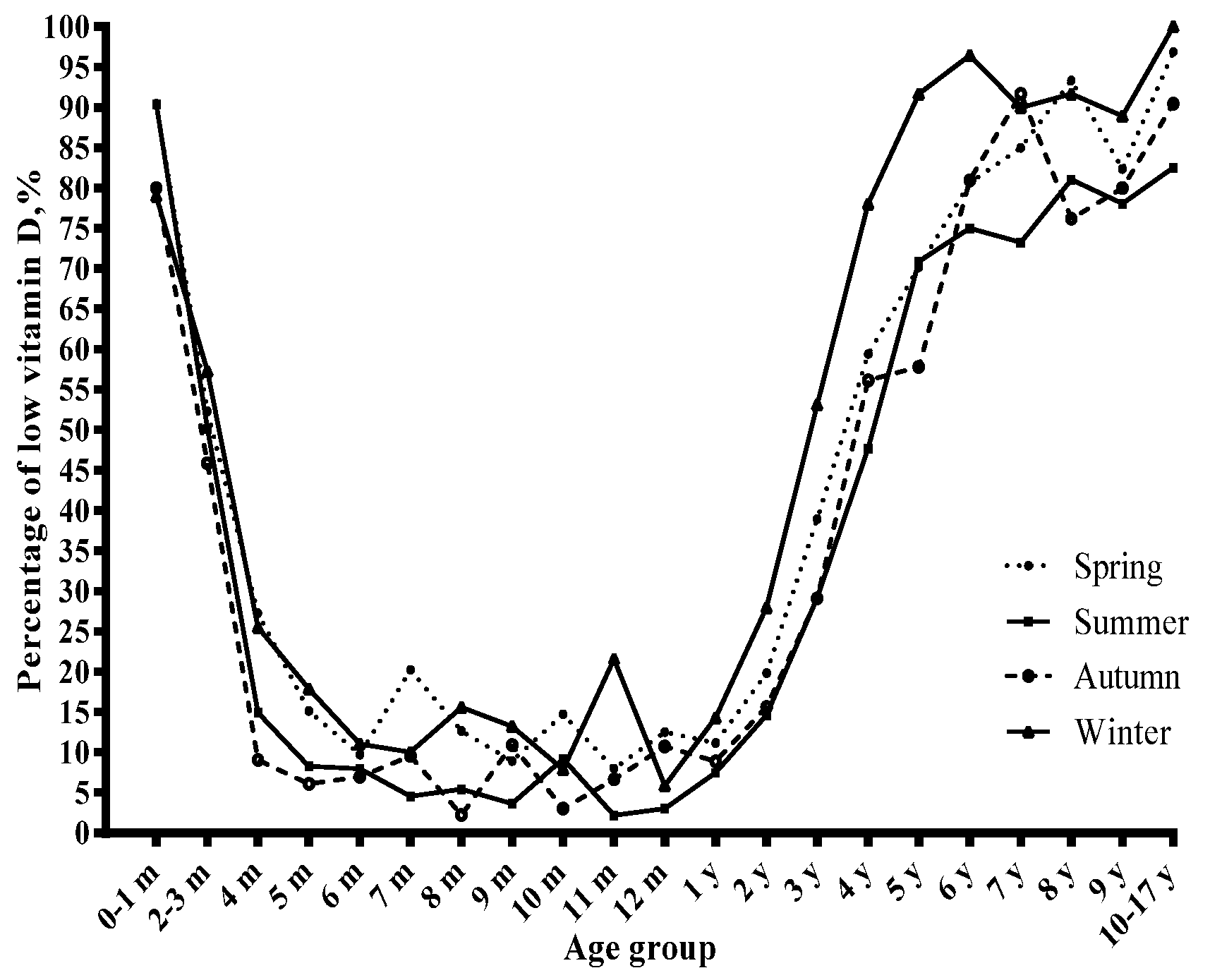
3.1. Age and Serum Vitamin D Status
3.2. Season and Serum Vitamin D Status
3.3. Visiting Type and Serum Vitamin D Status
3.4. Gender and Serum Vitamin D Status
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andiran, N.; Celik, N.; Akca, H.; Dogan, G. Vitamin D deficiency in children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2012, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Khor, G.L.; Chee, W.S.; Shariff, Z.M.; Poh, B.K.; Arumugam, M.; Rahman, J.A.; Theobald, H.E. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health 2011, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Ritu, G.; Gupta, A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients 2014, 6, 729–775. [Google Scholar]
- Zhao, X.; Xiao, J.; Liao, X.; Cai, L.; Xu, F.; Chen, D.; Xiang, J.; Fang, R. Vitamin D Status among Young Children Aged 1–3 Years: A Cross-Sectional Study in Wuxi, China. PLoS ONE 2015, 10, e0141595. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Laara, E.; Reunanen, A.; Jarvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Muhe, L.; Lulseged, S.; Mason, K.E.; Simoes, E.A. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 1997, 349, 1801–1804. [Google Scholar] [CrossRef]
- Mellati, A.A.; Sharifi, F.; Faghihzade, S.; Mousaviviri, S.A.; Chiti, H.; Kazemi, S.A. Vitamin D status and its associations with components of metabolic syndrome in healthy children. J. Pediatr. Endocrinol. Metab. 2015, 28, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, D.; Neupane, S.P.; Gundersen, T.E.; Madar, A.A. Vitamin D status in pre-school children in rural Nepal. Public Health Nutr. 2016, 19, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Crocombe, S.; McGrath, M.; Berry, J.L.; Mughal, M.Z. Hypovitaminosis D among healthy adolescent girls attending an inner city school. Arch. Dis. Child. 2006, 91, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; van den Hooven, E.H.; Heijboer, A.C.; Hofman, A.; Jaddoe, V.W.; Franco, O.H. Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J. Nutr. 2015, 145, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Lapatsanis, D.; Moulas, A.; Cholevas, V.; Soukakos, P.; Papadopoulou, Z.L.; Challa, A. Vitamin D: A necessity for children and adolescents in Greece. Calcif. Tissue Int. 2005, 77, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.R. Vitamin D-deficiency in Asia. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, W.; Wei, Z.; Ouyang, F.; Huang, L.; Wang, X.; Zhao, Y.; Zhang, H.; Zhang, J. Vitamin D status and related factors in newborns in Shanghai, China. Nutrients 2014, 6, 5600–5610. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.Y.; Qin, R.; Li, J.; Liang, G.X.; Guan, Y.J.; Gao, Z.H. Optimal level of 25-(OH)D in children in Nanjing (32 degrees N Lat) during winter. Pediatr. Int. 2011, 53, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Wang, H.Y.; Wen, H.K.; Tao, H.Q.; Zhao, X.W. Vitamin D status among infants, children, and adolescents in southeastern China. J. Zhejiang Univ. Sci. B 2016, 17, 545–552. [Google Scholar] [PubMed]
- Du, X.; Greenfield, H.; Fraser, D.R.; Ge, K.; Trube, A.; Wang, Y. Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am. J. Clin. Nutr. 2001, 74, 494–500. [Google Scholar] [PubMed]
- Wielders, J.P.; Carter, G.F.; Eberl, H.; Morris, G.; Roth, H.J.; Vogl, C. Automated Competitive Protein-Binding Assay for Total 25-OH Vitamin D, Multicenter Evaluation and Practical Performance. J. Clin. Lab. Anal. 2015, 29, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.K.; Tandon, N.; Reddy, D.R.; Aggarwal, R.; Singh, R.; Sawhney, R.C.; Saluja, B.; Ganie, M.A.; Singh, S. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am. J. Clin. Nutr. 2005, 82, 477–482. [Google Scholar] [PubMed]
- Strand, M.A.; Perry, J.; Jin, M.; Tracer, D.P.; Fischer, P.R.; Zhang, P.; Xi, W.; Li, S. Diagnosis of rickets and reassessment of prevalence among rural children in northern China. Pediatr. Int. 2007, 49, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhan, J.; Shao, J.; Chen, W.; Chen, L.; Li, W.; Ji, C.; Zhao, Z. High prevalence of vitamin D deficiency among children aged 1 month to 16 years in Hangzhou, China. BMC Public Health 2012, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency—Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [PubMed]
- Saki, F.; Dabbaghmanesh, M.H.; Omrani, G.R.; Bakhshayeshkaram, M. Vitamin D deficiency and its associated risk factors in children and adolescents in southern Iran. Public Health Nutr. 2015. [CrossRef] [PubMed]
- Salameh, K.; Al-Janahi, N.S.; Reedy, A.M.; Dawodu, A. Prevalence and risk factors for low vitamin D status among breastfeeding mother-infant dyads in an environment with abundant sunshine. Int. J. Womens Health 2016, 8, 529–535. [Google Scholar] [PubMed]
- Dawodu, A.; Davidson, B.; Woo, J.G.; Peng, Y.M.; Ruiz-Palacios, G.M.; De Lourdes Guerrero, M.; Morrow, A.L. Sun exposure and vitamin D supplementation in relation to vitamin D status of breastfeeding mothers and infants in the global exploration of human milk study. Nutrients 2015, 7, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Chinese Journal of Pediatrics. Recommendation for prevention and treatment of rickets of vitamin D deficiency in childhood. Chin. J. Pediatr. 2008, 46, 190–191. [Google Scholar]
- Valtuena, J.; Gonzalez-Gross, M.; Huybrechts, I.; Breidenassel, C.; Ferrari, M.; Mouratidou, T.; Gottrand, F.; Dallongeville, J.; Azzini, E.; Sioen, I.; et al. Factors associated with vitamin D deficiency in European adolescents: The HELENA study. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 161–171. [Google Scholar] [PubMed]
- Carpenter, T.O.; Herreros, F.; Zhang, J.H.; Ellis, B.K.; Simpson, C.; Torrealba-Fox, E.; Kim, G.J.; Savoye, M.; Held, N.A.; Cole, D.E. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am. J. Clin. Nutr. 2012, 95, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tuffaha, M.; El Bcheraoui, C.; Daoud, F.; Al Hussaini, H.A.; Alamri, F.; Al Saeedi, M.; Basulaiman, M.; Memish, Z.A.; AlMazroa, M.A.; Al Rabeeah, A.A.; et al. Deficiencies Under Plenty of Sun: Vitamin D Status among Adults in the Kingdom of Saudi Arabia, 2013. N. Am. J. Med. Sci. 2015, 7, 467–475. [Google Scholar] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin, D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]


| Variables | N (%) | Serum 25(OH)D | ||
|---|---|---|---|---|
| Mean ± SD | β (se) | p Value | ||
| Gender | ||||
| Boys | 7739 (55.3) | 39 ± 12 | REF | |
| Girls | 6258 (44.7) | 40 ± 12 | 0.13(0.19) | 0.50 |
| Age | ||||
| 0–3 months | 1288 (9.2) | 29 ± 11 | REF | |
| 4–6 months | 2162 (15.5) | 43 ± 11 | 13.27(0.42) | <0.01 |
| 7–12 months | 1577 (11.3) | 45 ± 11 | 15.29(0.44) | <0.01 |
| 1–3 years | 7703 (55.0) | 41 ± 11 | 11.27(0.39) | <0.01 |
| 4–6 years | 786 (5.6) | 27 ± 8 | −3.25(0.45) | <0.01 |
| 7–10 years | 395 (2.8) | 23 ± 7 | −6.79(0.50) | <0.01 |
| 11–17 years | 86 (0.6) | 21 ± 8 | −8.97(0.87) | <0.01 |
| Season | ||||
| Spring | 3919 (28.0) | 39 ± 12 | REF | |
| Summer | 4914 (35.1) | 40 ± 12. | 1.62(0.23) | <0.01 |
| Autumn | 2451 (17.5) | 40 ± 12 | 1.13(0.27) | <0.01 |
| Winter | 2713 (19.4) | 36 ± 12 | −2.56(0.27) | <0.01 |
| Visiting Type | ||||
| Health Examination | 8499 (60.7) | 41 ± 11 | REF | |
| Clinical Visiting | 5498 (39.3) | 37 ± 13 | −0.45(0.21) | 0.03 |
| Variables | Sufficiency | Insufficiency | Deficiency | ||||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95%CI) | p Value | N (%) | OR(95%CI) | p Value | |
| Gender | |||||||
| Boys | 5874 (54.7) | 1364 (57.9) | REF | 501 (55.7) | REF | ||
| Girls | 4865 (45.3) | 994 (42.2) | 0.96 (0.87-1.05) | 0.35 | 399 (44.3) | 1.14 (0.98-1.33) | 0.10 |
| Age | |||||||
| 0–3 months | 570 (5.3) | 424 (18.0) | REF | 294 (32.7) | REF | ||
| 4–6 months | 1926 (17.9) | 179 (7.6) | 0.14 (0.11–0.17) | <0.01 | 57 (6.3) | 0.06 (0.05–0.09) | <0.01 |
| 7–12 months | 1436 (13.4) | 114 (4.8) | 0.12 (0.10–0.15) | <0.01 | 27 (3.0) | 0.04 (0.03–0.06) | <0.01 |
| 1–3 years | 6466 (60.2) | 1064 (45.1) | 0.26 (0.22–0.31) | <0.01 | 173 (19.2) | 0.06 (0.05–0.08) | <0.01 |
| 4–6 years | 264 (2.5) | 352 (14.9) | 2.14 (1.74–2.64) | <0.01 | 170 (18.9) | 1.66 (1.29–2.14) | <0.01 |
| 7–10 years | 70 (0.6) | 187 (7.9) | 4.32 (3.18–5.87) | <0.01 | 138 (15.3) | 5.31 (3.81–7.41) | <0.01 |
| 11–17 years | 7 (0.1) | 38 (1.6) | 8.57 (3.78–19.44) | <0.01 | 41 (4.6) | 14.61 (6.41–33.27) | <0.01 |
| Season | |||||||
| Spring | 3004 (28.0) | 638 (27.0) | REF | 277 (30.8) | REF | ||
| Summer | 3864 (36.0) | 796 (33.8) | 0.78 (0.69–0.89) | <0.01 | 254 (28.2) | 0.49 (0.41–0.60) | <0.01 |
| Autumn | 1964 (18.3) | 371 (15.7) | 0.80 (0.69–0.93) | <0.01 | 116 (12.9) | 0.53 (0.42–0.68) | <0.01 |
| Winter | 1907 (17.7) | 553 (23.5) | 1.37 (1.20–1.58) | <0.01 | 253 (28.1) | 1.33 (1.09–1.63) | 0.01 |
| Visiting Type | |||||||
| Health Examination | 7005 (65.2) | 1170 (49.6) | REF | 324 (36.0) | REF | ||
| Clinical Visiting | 3734 (34.8) | 1188 (50.4) | 1.19 (1.07–1.33) | <0.01 | 576 (64.0) | 1.23 (1.03–1.47) | 0.03 |
| Variables | Distribution of Serum 25(OH)D | p Value | ||
|---|---|---|---|---|
| Sufficiency (n = 10,739) | Insufficiency (n = 2358) | Deficiency (n = 900) | ||
| N (%) | ||||
| Gender a | ||||
| Boys | 5874 (75.9) | 1364 (17.6) | 501 (6.5) | 0.02 |
| Girls | 4865 (77.7) | 994 (15.9) | 399 (6.4) | |
| Season a | ||||
| Spring | 3004 (76.6) | 638 (16.3) | 277 (7.1) | <0.01 |
| Summer | 3864 (78.6) | 796 (16.2) | 254 (5.2) | |
| Autumn | 1964 (80.2) | 371 (15.1) | 116 (4.7) | |
| Winter | 1907 (70.3) | 553 (20.4) | 253 (9.3) | |
| Visiting Type a | ||||
| Health Examination | 7005 (82.4) | 1170 (13.8) | 324 (3.8) | <0.01 |
| Clinical Visiting | 3734 (67.9) | 1188 (21.6) | 576 (10.5) | |
| Mean ± SD | ||||
| Age, years b | 3 ± 4 | 3 ± 3 | 1 ± 1 | <0.01 |
| 25(OH)D, ng/mL b | 44 ± 9 | 26 ± 3 | 1 ± 4 | <0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Shen, G.; Jiang, S.; Xu, H.; Li, M.; Wang, Z.; Zhang, S.; Yu, Y. Nutrient Status of Vitamin D among Chinese Children. Nutrients 2017, 9, 319. https://doi.org/10.3390/nu9040319
Wang S, Shen G, Jiang S, Xu H, Li M, Wang Z, Zhang S, Yu Y. Nutrient Status of Vitamin D among Chinese Children. Nutrients. 2017; 9(4):319. https://doi.org/10.3390/nu9040319
Chicago/Turabian StyleWang, Shuojia, Guosong Shen, Shuying Jiang, Hongwei Xu, Minchao Li, Zhaopin Wang, Su Zhang, and Yunxian Yu. 2017. "Nutrient Status of Vitamin D among Chinese Children" Nutrients 9, no. 4: 319. https://doi.org/10.3390/nu9040319




