Anti‐Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Cell Viability
2.3. Adhesion Assay
2.4. Wound-Healing Assay
2.5. Transwell-Migration Assay
2.6. Western Blot
2.7. Real Time RT-PCR
2.8. Animal Study
2.9. Isolation of Major Compounds of PFE and UPLC Analysis
2.10. Statistical Analysis
3. Results
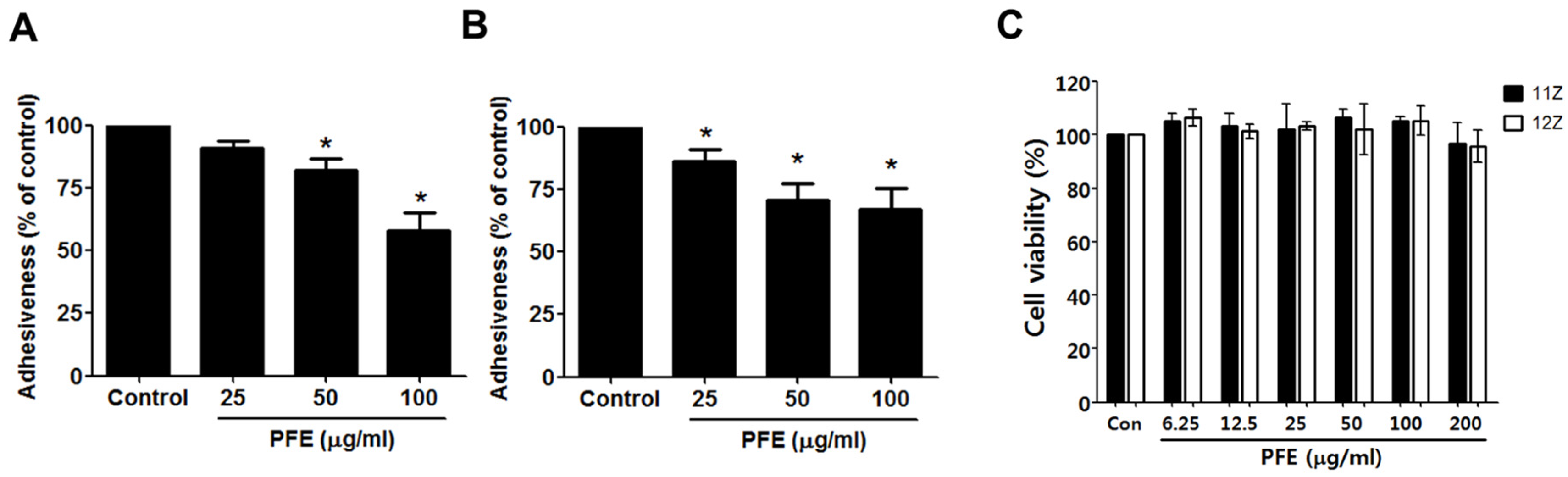
3.1. PFE Treatment Inhibits Endometriotic Cell Adhesion to Mesothelial Cells
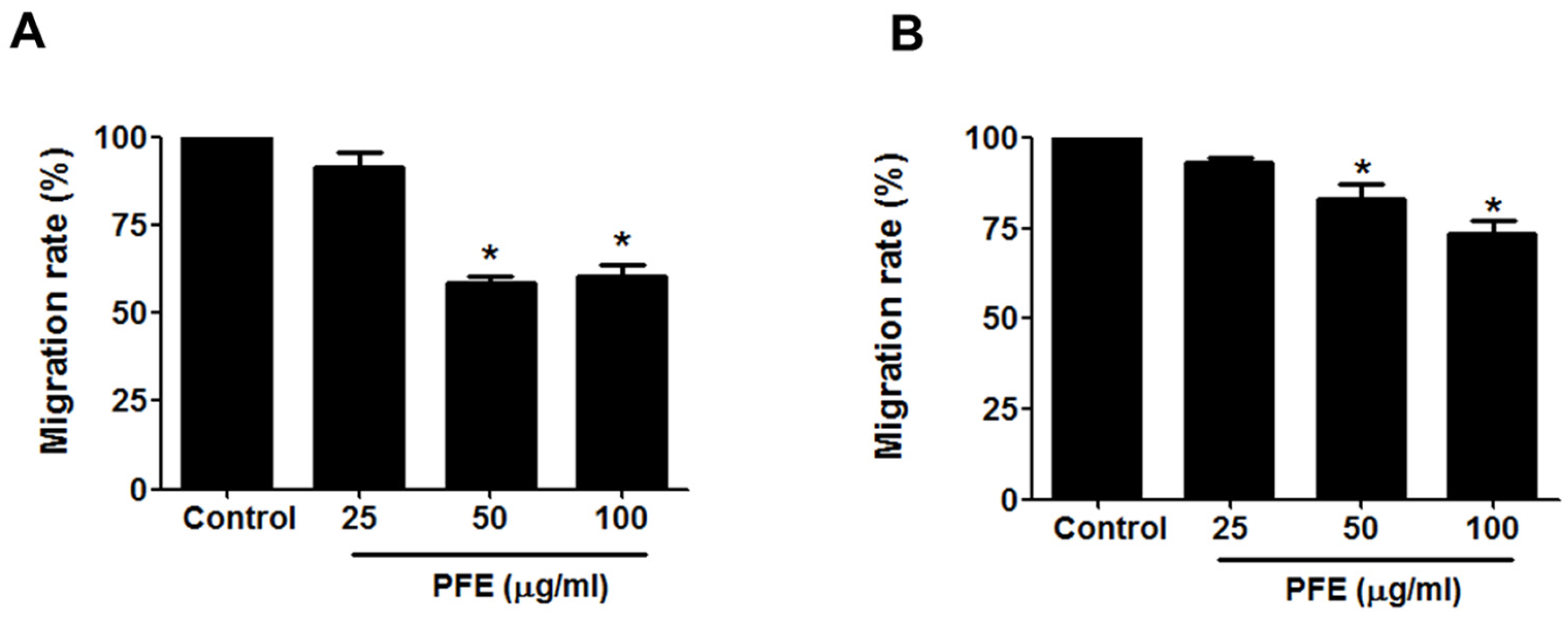
3.2. PFE Inhibits Endometriotic Cell Migration
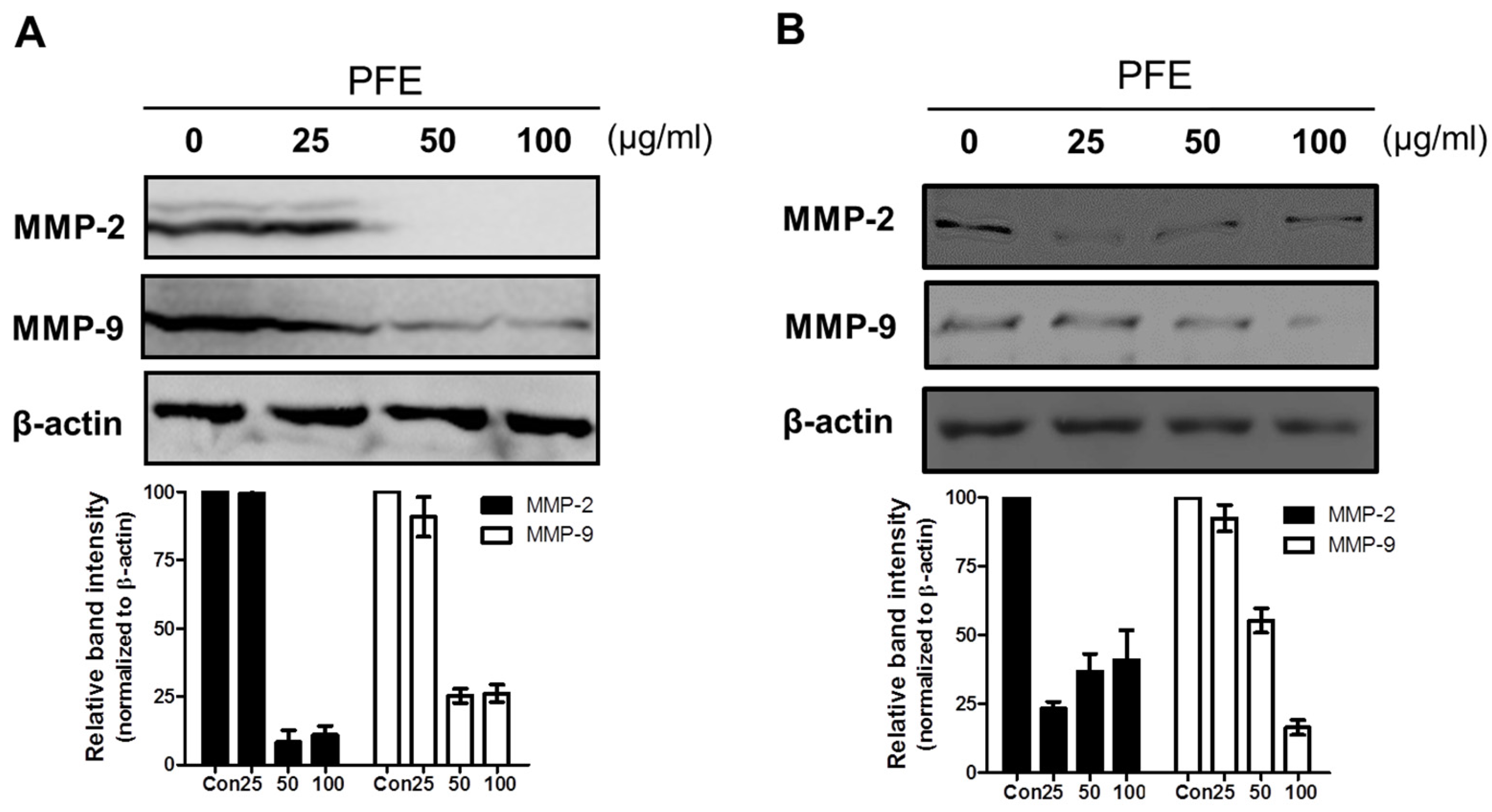
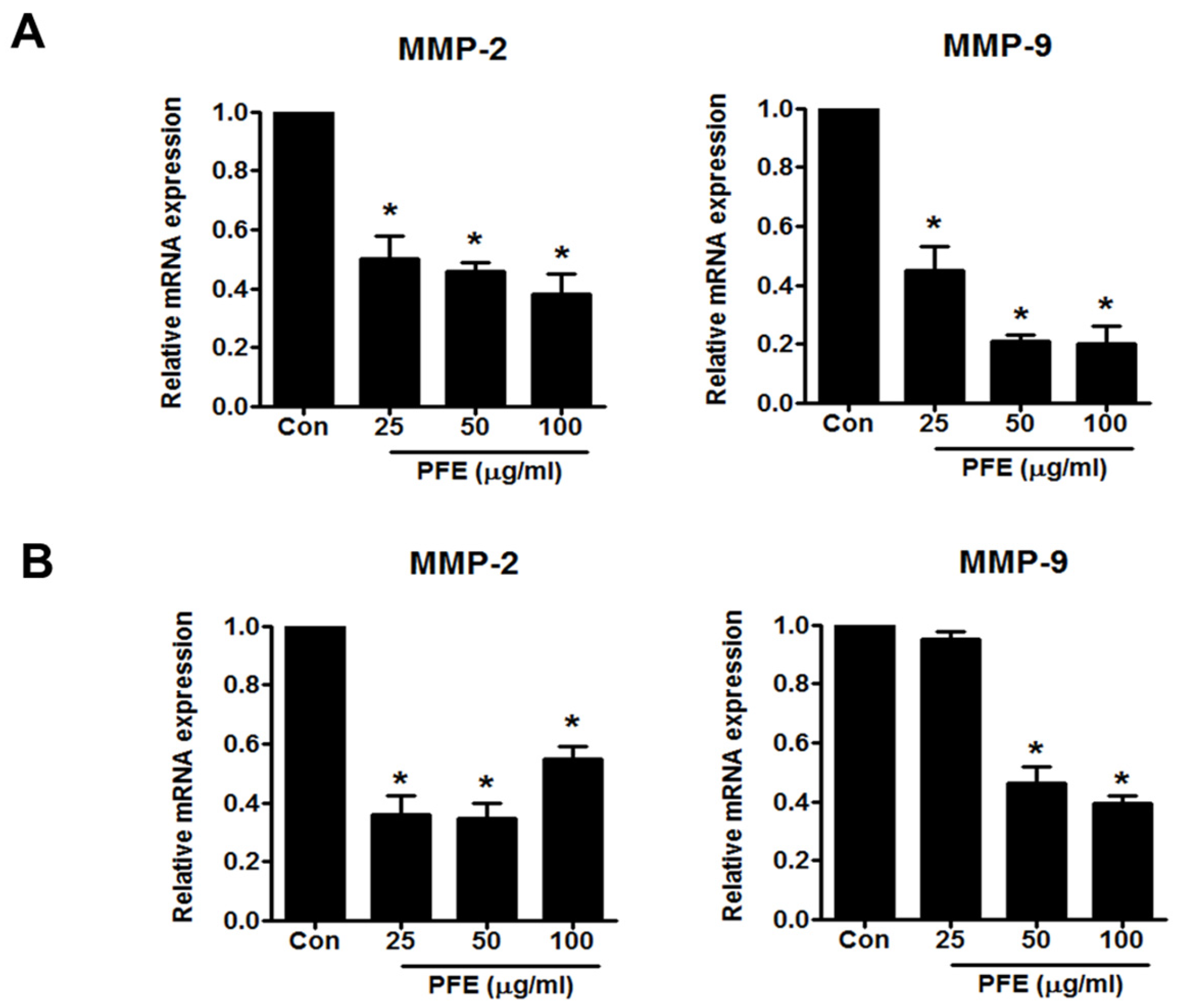
3.3. PFE Inhibits the Expression of MMP-2 and MMP-9 in Human Endometriotic Cells
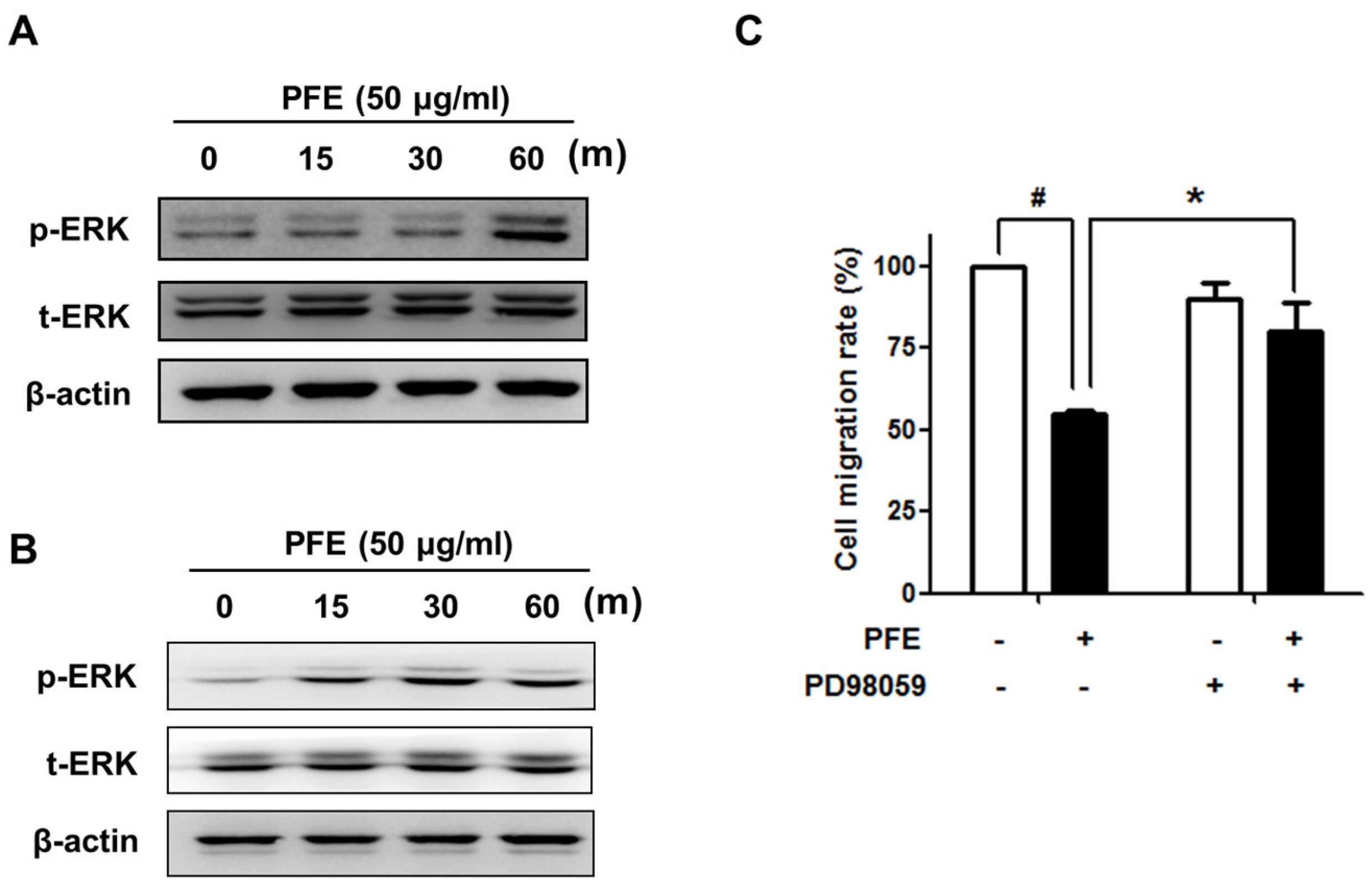
3.4. ERK Signalling Is Involved in PFE-Induced Anti-Endometriotic Activity
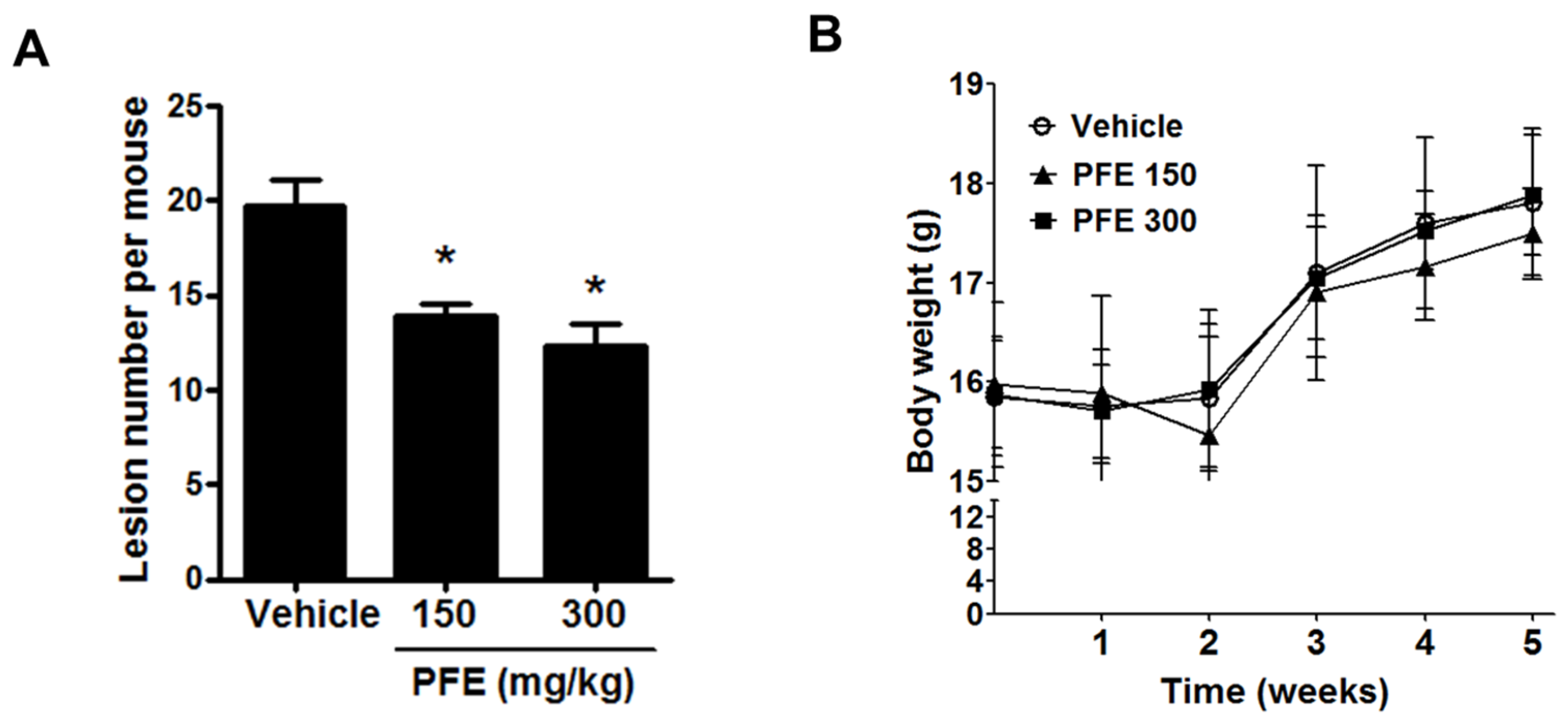
3.5. PFE Inhibits the Formation of Endometriosis-Like Lesions in Mice
3.6. Identification of Major Compounds of PFE and Anti-Endometriotic Effect of Tectorigenin
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Chung, H.W.; Wen, Y.; Chun, S.H.; Nezhat, C.; Woo, B.H.; Lake Polan, M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mrna expression in ectopic and eutopic endometrium in women with endometriosis: A rationale for endometriotic invasiveness. Fertil. Steril. 2001, 75, 152–159. [Google Scholar] [CrossRef]
- Osteen, K.G.; Yeaman, G.R.; Bruner-Tran, K.L. Matrix metalloproteinases and endometriosis. Semin. Reprod. Med. 2003, 21, 155–164. [Google Scholar] [PubMed]
- Wenzl, R.J.; Heinzl, H. Localization of matrix metalloproteinase-2 in uterine endometrium and ectopic implants. Gynecol. Obstet. Investig. 1998, 45, 253–257. [Google Scholar] [CrossRef]
- Garai, J.; Molnar, V.; Varga, T.; Koppan, M.; Torok, A.; Bodis, J. Endometriosis: Harmful survival of an ectopic tissue. Front. Biosci. 2006, 11, 595–619. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2016; Volume 10. [Google Scholar]
- Blaustein, R. Kudsu’s invasion into southern united states life and culture. In The Great Reshuffling: Human Dimensions of Invasive Species; McNeelley, J.A., Ed.; The World Conservation Union: Cambridge, UK, 2001; pp. 55–62. [Google Scholar]
- Keung, W.M.; Vallee, B.L. Kudzu root: An ancient chinese source of modern antidipsotropic agents. Phytochemistry 1998, 47, 499–506. [Google Scholar] [CrossRef]
- Woo, J.; Lau, E.; Ho, S.C.; Cheng, F.; Chan, C.; Chan, A.S.; Haines, C.J.; Chan, T.Y.; Li, M.; Sham, A. Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause 2003, 10, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Bae, E.A.; Kim, D.H. Hepatoprotective effect of tectoridin and tectorigenin on tert-butyl hyperoxide-induced liver injury. J. Pharmacol. Sci. 2005, 97, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Park, Y.J.; Kim, D.H. Kakkalide and its metabolite irisolidone ameliorate carrageenan-induced inflammation in mice by inhibiting nf-kappab pathway. Inflammation 2011, 34, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Sohn, I.C.; Kim, D.H.; Choi, J.W.; Kwon, S.H.; Park, H.J. Hypoglycemic and hypolipidemic effects of tectorigenin and kaikasaponin iii in the streptozotocin-lnduced diabetic rat and their antioxidant activity in vitro. Arch. Pharm. Res. 2000, 23, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Sohn, I.C.; Kim, Y.K.; Choi, J.H.; Choi, J.W.; Park, H.J.; Itoh, Y.; Miyamoto, K. Tectorigenin, an isoflavone of pueraria thunbergiana benth., induces differentiation and apoptosis in human promyelocytic leukemia hl-60 cells. Biol. Pharm. Bull. 2001, 24, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jung, G.O.; Choi, J.; Lee, K.T.; Park, H.J. Potent antimutagenic and their anti-lipid peroxidative effect of kaikasaponin iii and tectorigenin from the flower of pueraria thunbergiana. Arch. Pharm. Res. 2002, 25, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Bae, E.A.; Lee, Y.C.; Ma, J.Y.; Kim, D.H. Estrogenic effect of main components kakkalide and tectoridin of puerariae flos and their metabolites. Biol. Pharm. Bull. 2006, 29, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Cherdshewasart, W.; Sutjit, W.; Pulcharoen, K.; Chulasiri, M. The mutagenic and antimutagenic effects of the traditional phytoestrogen-rich herbs, pueraria mirifica and pueraria lobata. Braz. J. Med. Biol. Res. 2009, 42, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.H.; Kim, T.; Ahn, S.; Kim, Y.J.; Kim, H.Y.; Piao, X.L.; Park, J.H. Evaluation of the estrogenic activity of leguminosae plants. Biol. Pharm. Bull. 2005, 28, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an invasive, n-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am. J. Pathol. 2001, 159, 1839–1852. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, B.; Yang, Q.; Fearns, C.; Yates, J.R., 3rd; Lee, J.D. Combined integrin phosphoproteomic analyses and small interfering RNA—Based functional screening identify key regulators for cancer cell adhesion and migration. Cancer Res. 2009, 69, 3713–3720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, W.; Pu, D.; Li, Z.; Huang, Q.; Chen, Q. The inhibitory effect of 15-r-lxa4 on experimental endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Harada, M.; Takemura, Y.; Morimoto, C.; Koga, K.; Yano, T.; Tsutsumi, O.; et al. Development of an experimental model of endometriosis using mice that ubiquitously express green fluorescent protein. Hum. Reprod. 2005, 20, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wang, Q.; Fu, X.H.; Huang, X.H.; Chen, X.L.; Cao, L.Q.; Chen, L.Z.; Tan, H.X.; Li, W.; Bi, J.; et al. Involvement of pi3k/pten/akt/mtor pathway in invasion and metastasis in hepatocellular carcinoma: Association with mmp-9. Hepatol. Res. 2009, 39, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, Y.G.; Pu, D.M. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol. Obstet. Investig. 2006, 62, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of mapk signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, F.; Guo, S.; Ding, F.; He, Y.; Wu, J.; Liu, C. Protective effect of flos puerariae extract following acute alcohol intoxication in mice. Alcohol. Clin. Exp. Res. 2014, 38, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Chen, H.G.; Wu, P.F.; Yao, Q.; Cheng, H.K.; Yu, W.; Liu, C. Flos puerariae extract ameliorates cognitive impairment in streptozotocin-induced diabetic mice. Evid. Based Complement. Alternat. Med. 2015, 2015, 873243. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Sameshima-Kamiya, M.; Nagamine, R.; Tsubata, M.; Ikeguchi, M.; Takagaki, K.; Shimada, T.; Aburada, M. The crude extract from puerariae flower exerts antiobesity and antifatty liver effects in high-fat diet-induced obese mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 272710. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.K.; Lee, J.; Speights, V.O., Jr.; Starzinski-Powitz, A.; Arosh, J.A. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology 2008, 149, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.E.; Nothnick, W.B. The relevancy of the matrix metalloproteinase system to the pathophysiology of endometriosis. Front. Biosci. 2005, 10, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Styer, A.K.; Sullivan, B.T.; Puder, M.; Arsenault, D.; Petrozza, J.C.; Serikawa, T.; Chang, S.; Hasan, T.; Gonzalez, R.R.; Rueda, B.R. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology 2008, 149, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lee, J.Y.; Moon, H.S.; Hur, S.E.; Park, M.H.; Wen, Y.; Polan, M.L. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil. Steril. 2002, 78, 787–795. [Google Scholar] [CrossRef]
- Huang, H.F.; Hong, L.H.; Tan, Y.; Sheng, J.Z. Matrix metalloproteinase 2 is associated with changes in steroid hormones in the sera and peritoneal fluid of patients with endometriosis. Fertil. Steril. 2004, 81, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Edelstein, L.C.; Chen, C.; Bash, J.; Gelinas, C. The prosurvival bcl-2 homolog bfl-1/a1 is a direct transcriptional target of nf-kappab that blocks tnfalpha-induced apoptosis. Genes Dev. 1999, 13, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Malberg, K.; Arndt, M.; Schmitt, J.; Roessner, A.; Schultze, D.; Kleinstein, J.; Ansorge, S. Matrix metalloproteinases and tace play a role in the pathogenesis of endometriosis. Adv. Exp. Med. Biol. 2000, 477, 483–486. [Google Scholar] [PubMed]
- Aznaurova, Y.B.; Zhumataev, M.B.; Roberts, T.K.; Aliper, A.M.; Zhavoronkov, A.A. Molecular aspects of development and regulation of endometriosis. Reprod. Biol. Endocrinol. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.D.; Kocbek, V.; Nirgianakis, K.; Bersinger, N.A.; Mueller, M.D. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum. Reprod. Update 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, D.; Busacca, M.; Di Francesco, S.; Vignali, M.; Vigano, P.; Di Blasio, A.M. Pi3k/akt and erk1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol. Hum. Reprod. 2007, 13, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Chaiwangyen, W.; Ospina-Prieto, S.; Morales-Prieto, D.M.; Pereira de Sousa, F.L.; Pastuschek, J.; Fitzgerald, J.S.; Schleussner, E.; Markert, U.R. Oncostatin m and leukaemia inhibitory factor trigger signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2 pathways but result in heterogeneous cellular responses in trophoblast cells. Reprod. Fertil. Dev. 2016, 28, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wu, X.C.; Huang, W. Raloxifene upregulated mesangial cell mmp-2 activity via er-beta through transcriptional regulation. Cell Biochem. Biophys. 2013, 67, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, M.; Bai, J.; Liu, Q.; Xi, Y.; Li, W.; Zheng, J. Role of runx3 in suppressing metastasis and angiogenesis of human prostate cancer. PLoS ONE 2014, 9, e86917. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liao, Y.; Li, G.Q.; Law, F.C.; Tang, Y. Quantitative analysis of two isoflavones in pueraria lobata flowers from eleven Chinese provinces using high performance liquid chromatography. Chin. Med. 2010, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Shin, S.; Ha, H.; Kim, J.M. Study of substance changes in flowers of pueraria thunbergiana benth. During storage. Arch. Pharm. Res. 2003, 26, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Bae, J.Y.; Lee, Y.J.; Ahn, M.J. Tectoridin from maackia amurensis modulates both estrogen and thyroid receptors. Phytomedicine 2014, 21, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.; Yamada, M.; Lim, S.S.; Lee, S.H.; Ryu, N.; Shin, K.H.; Ohuchi, K. Inhibition by tectorigenin and tectoridin of prostaglandin e2 production and cyclooxygenase-2 induction in rat peritoneal macrophages. Biochim. Biophys. Acta 1999, 1438, 399–407. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, Y.S.; Lee, S.; Lim, S.S.; Kim, Y.S.; Ohuchi, K.; Shin, K.H. Anti-angiogenic and anti-tumor activities of isoflavonoids from the rhizomes of belamcanda chinensis. Planta Med. 2003, 69, 617–622. [Google Scholar] [PubMed]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, Y.; Yang, J.; Chai, H.; Li, Y.; Yang, J.; Jia, Z.; Wang, Z. Tectoridin, an isoflavone glycoside from the flower of pueraria lobata, prevents acute ethanol-induced liver steatosis in mice. Toxicology 2010, 276, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, R.; Kodera, M.; Sakamoto, S.; Nakajima, Y.; Yamazaki, T.; Niiho, Y.; Nohara, T.; Kinjo, J. Microbial transformation and bioactivation of isoflavones from pueraria flowers by human intestinal bacterial strains. J. Nat. Med. 2009, 63, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.H.; Cheng, Z.H.; Ou-Yang, H.N.; Luo, C.; Guo, Z.L. Tectorigenin inhibits osteosarcoma cell migration through downregulation of matrix metalloproteinases in vitro. Anticancer Drugs 2016, 27, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Kim, S.Y.; Kim, H.S.; Kim, D.H.; Hyun, J.W. Cytoprotective effect of tectorigenin, a metabolite formed by transformation of tectoridin by intestinal microflora, on oxidative stress induced by hydrogen peroxide. Eur. J. Pharmacol. 2005, 519, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, S.B.; Jung, S.H.; Cha, K.H.; Park, W.D.; Sohn, Y.C.; Nho, C.W. Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an erk-dependent pathway. Mol. Cells 2009, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Woo, J.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J. Anti‐Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice. Nutrients 2017, 9, 212. https://doi.org/10.3390/nu9030212
Kim J, Woo J, Kim HM, Oh MS, Jang DS, Choi J. Anti‐Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice. Nutrients. 2017; 9(3):212. https://doi.org/10.3390/nu9030212
Chicago/Turabian StyleKim, Ji‐Hyun, Jeong‐Hwa Woo, Hye Mi Kim, Myung Sook Oh, Dae Sik Jang, and Jung‐Hye Choi. 2017. "Anti‐Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice" Nutrients 9, no. 3: 212. https://doi.org/10.3390/nu9030212




