The Effects of Iodine Fortified Milk on the Iodine Status of Lactating Mothers and Infants in an Area with a Successful Salt Iodization Program: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Intervention
2.3. Urine and Milk Samples Collection
2.4. Salt Sample Collection
2.5. Laboratory Measurements
2.6. Definition
2.7. Statistics Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delange, F. The role of iodine in brain development. Proc. Nutr. Soc. 2000, 59, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Delange, F. Iodine deficiency as a cause of brain damage. Postgrad. Med. J. 2001, 77, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: A review. Thyroid 2007, 17, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The effects of iodine deficiency in pregnancy and infancy. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. S1), 108–117. [Google Scholar] [CrossRef] [PubMed]
- United Nations International Children’s Emergency Fund (UNICEF). The State of the World’s Children 2009: Maternal and Newborn Health: Unicef; UNICEF: New York, NY, USA, 2008. [Google Scholar]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. 2007. Available online: whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf (accessed on 30 October 2016).
- World Health Organization. Salt as a Vehicle for Fortification; Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Charlton, K.E.; Jooste, P.L.; Steyn, K.; Levitt, N.S.; Ghosh, A. A lowered salt intake does not compromise iodine status in Cape Town, South Africa, where salt iodization is mandatory. Nutrition 2013, 29, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Johner, S.A.; Gunther, A.L.; Remer, T. Current trends of 24-h urinary iodine excretion in German schoolchildren and the importance of iodised salt in processed foods. Br. J. Nutr. 2011, 106, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, P.; Mirmiran, P.; Mehrabi, Y.; Hedayati, M.; Delshad, H.; Azizi, F. Evaluation of iodine nutritional status in Tehran, Iran: Iodine deficiency within iodine sufficiency. Thyroid 2010, 20, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Tayie, F.A.; Jourdan, K. Hypertension, dietary salt restriction, and iodine deficiency among adults. Am. J. Hypertens. 2010, 23, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Aeberli, I.; Wust, N.; Piacenza, A.M.; Bucher, T.; Henschen, I.; Haldimann, M.; Zimmermann, M.B. The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J. Clin. Endocrinol. Metab. 2010, 95, 5217–5224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brough, L.; Jin, Y.; Shukri, N.H.; Wharemate, Z.R.; Weber, J.L.; Coad, J. Iodine intake and status during pregnancy and lactation before and after government initiatives to improve iodine status, in Palmerston North, New Zealand: A pilot study. Matern. Child Nutr. 2015, 11, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Verd, S.; Aramburu, A.; Carreras, G. Iodine supplementation for lactation: Time for tailoring treatments targeted to specific subgroups. J. Paediatr. Child Health 2013, 49, E353–E354. [Google Scholar] [CrossRef] [PubMed]
- Cressey, P. Iodine content of New Zealand dairy products. J. Food Comp. Anal. 2003, 16, 25–36. [Google Scholar] [CrossRef]
- Dahl, L.; Johansson, L.; Julshamn, K.; Meltzer, H.M. The iodine content of Norwegian foods and diets. Public Health Nutr. 2004, 7, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Guyot, H.; Saegerman, C.; Lebreton, P.; Sandersen, C.; Rollin, F. Epidemiology of trace elements deficiencies in Belgian beef and dairy cattle herds. J. Trace Elem. Med. Biol. 2009, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Johner, S.A.; Thamm, M.; Nothlings, U.; Remer, T. Iodine status in preschool children and evaluation of major dietary iodine sources: A German experience. Eur. J. Nutr. 2013, 52, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Waite, K.V.; Ma, G.; Eastman, C.J. Declining iodine content of milk and re-emergence of iodine deficiency in Australia. Med. J. Aust. 2006, 184, 307. [Google Scholar] [PubMed]
- Pearce, E.N.; Pino, S.; He, X.; Bazrafshan, H.R.; Lee, S.L.; Braverman, L.E. Sources of dietary iodine: Bread, cows’ milk, and infant formula in the Boston area. J. Clin. Endocrinol. Metab. 2004, 89, 3421–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, L.B.; Ovesen, L.; Bulow, I.; Jorgensen, T.; Knudsen, N.; Laurberg, P.; Pertild, H. Dietary iodine intake and urinary iodine excretion in a Danish population: Effect of geography, supplements and food choice. Br. J. Nutr. 2002, 87, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; McLean, R.; Davies, B.; Hawkins, R.; Meiklejohn, E.; Ma, Z.F.; Skeaff, S. Adequate Iodine Status in New Zealand School Children Post-Fortification of Bread with Iodised Salt. Nutrients 2016, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Girelli, M.E.; Coin, P.; Mian, C.; Nacamulli, D.; Zambonin, L.; Piccolo, M.; Vianello-Dri, A.; Gottardo, F.; Busnardo, B. Milk represents an important source of iodine in schoolchildren of the Veneto region, Italy. J. Endocrinol. Investig. 2004, 27, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Gutierrez-Repiso, C.; Gonzalez-Romero, S.; Olveira, G.; Garriga, M.J.; Velasco, I.; Santiago, P.; de Escobar, G.M.; Garcia-Fuentes, E. Iodine concentration in cow’s milk and its relation with urinary iodine concentrations in the population. Clin. Nutr. 2011, 30, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Mehran, L.; Sheikholeslam, R.; Ordookhani, A.; Naghavi, M.; Hedayati, M.; Padyab, M.; Mirmiran, P. Sustainability of a well-monitored salt iodization program in Iran: Marked reduction in goiter prevalence and eventual normalization of urinary iodine concentrations without alteration in iodine content of salt. J. Endocrinol. Investig. 2008, 31, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Sheikholeslam, R.; Hedayati, M.; Mirmiran, P.; Malekafzali, H.; Kimiagar, M.; Pajouhi, M. Sustainable control of iodinedeficiency in Iran: Beneficial results of the implementation of the mandatory law on salt iodization. J. Endocrinol. Investig. 2002, 25, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef] [PubMed]
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef] [PubMed]
- Norouzian, M.; Valizadeh, R.; Azizi, F.; Hedayati, M.; Naserian, A.; Shahroodi, F.E. The effect of feeding different levels of potassium iodide on performance, T3 and T4 concentrations and iodine excretion in Holstein dairy cows. J. Anim. Vet. Adv. 2009, 8, 111–114. [Google Scholar]
- Norouzian, M.A. Iodine in raw and pasteurized milk of dairy cows fed different amounts of potassium iodide. Biol. Trace Elem. Res. 2011, 139, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.; Khazan, M.; Yaghmaee, P.; Yeghaneh, M.Z.; Behdadfar, L.; Daneshpour, M.S. Rapid microwave digestion and microplate reading format method for urinary iodine determination. Clin. Chem. Lab. Med. 2011, 49, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.; Ordookhani, A.; Daneshpour, M.S.; Azizi, F. Rapid acid digestion and simple microplate method for milk iodine determination. J. Clin. Lab. Anal. 2007, 21, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Smyth, P. Breastfeeding and maternal and infant iodine nutrition. Clin. Endocrinol. (Oxf.) 2009, 70, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Button, S.; Rayman, M.P. Iodine concentration of organic and conventional milk: Implications for iodine intake. Br. J. Nutr. 2012, 107, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Watutantrige Fernando, S.; Barollo, S.; Nacamulli, D.; Pozza, D.; Giachetti, M.; Frigato, F.; Redaelli, M.; Zagotto, G.; Girelli, M.E.; Mantero, F.; et al. Iodine status in schoolchildren living in northeast Italy: The importance of iodized-salt use and milk consumption. Eur. J. Clin. Nutr. 2013, 67, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Carle, A.; Jorgensen, T.; Knuthsen, P.; Krejbjerg, A.; Perrild, H.; Bjergved, L.; Sloth, J.J.; Laurberg, P.; Ovesen, L. Iodine excretion has decreased in Denmark between 2004 and 2010—The importance of iodine content in milk. Br. J. Nutr. 2014, 112, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Payling, L.M.; Juniper, D.T.; Drake, C.; Rymer, C.; Givens, D.I. Effect of milk type and processing on iodine concentration of organic and conventional winter milk at retail: Implications for nutrition. Food Chem. 2015, 178, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, A. Iodine Determination in Raw Cow’s Milk in Iran. JFBT 2014, 4, 13–20. [Google Scholar]
- Rasmussen, L.B.; Carle, A.; Jorgensen, T.; Knudsen, N.; Laurberg, P.; Pedersen, I.B.; Perrild, H.; Vejbjerg, P.; Ovesen, L. Iodine intake before and after mandatory iodization in Denmark: Results from the Danish Investigation of Iodine Intake and Thyroid Diseases (DanThyr) study. Br. J. Nutr. 2008, 100, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Jorgensen, T.; Perrild, H.; Knudsen, N.; Krejbjerg, A.; Laurberg, P.; Pedersen, I.B.; Bjergved, L.; Ovesen, L. Mandatory iodine fortification of bread and salt increases iodine excretion in adults in Denmark—A 11-year follow-up study. Clin. Nutr. 2014, 33, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Abel, M.H.; Haugen, M.; Meltzer, H.M. Risk of suboptimal iodine intake in pregnant Norwegian women. Nutrients 2013, 5, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Haugen, M.; Julshamn, K.; Alexander, J.; Meltzer, H.M. Evaluation of urinary iodine excretion as a biomarker for intake of milk and dairy products in pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Eur. J. Clin. Nutr. 2009, 63, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsdottir, I.; Gustavsdottir, A.G.; Steingrimsdottir, L.; Maage, A.; Johannesson, A.J.; Thorsdottir, I. Iodine status of pregnant women in a population changing from high to lower fish and milk consumption. Public Health Nutr. 2013, 16, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Perrine, C.G.; Herrick, K.; Serdula, M.K.; Sullivan, K.M. Some subgroups of reproductive age women in the United States may be at risk for iodine deficiency. J. Nutr. 2010, 140, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Untoro, J.; Mangasaryan, N.; de Benoist, B.; Darnton-Hill, I. Reaching optimal iodine nutrition in pregnant and lactating women and young children: Programmatic recommendations. Public Health Nutr. 2007, 10, 1527–1529. [Google Scholar] [CrossRef] [PubMed]
- Aakre, I.; Strand, T.A.; Bjøro, T.; Norheim, I.; Barikmo, I.; Ares, S.; Alcorta, M.D.; Henjum, S. Thyroid Function among Breastfed Children with Chronically Excessive Iodine Intakes. Nutrients 2016, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Ohashi, T.; Yamaguchi, S.; Yamaguchi, M.; Shishiba, Y.; Irie, M. Iodine status of pregnant and postpartum Japanese women: Effect of iodine intake on maternal and neonatal thyroid function in an iodine-sufficient area. J. Clin. Endocrinol. Metab. 2011, 96, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sang, Z.; Tan, L.; Zhang, S.; Dong, F.; Chu, Z.; Wei, W.; Zhao, N.; Zhang, G.; Yao, Z.; et al. Neonatal thyroid function born to mothers living with long-term excessive iodine intake from drinking water. Clin. Endocrinol. (Oxf.) 2015, 83, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Nepal, A.K.; Suwal, R.; Gautam, S.; Shah, G.S.; Baral, N.; Andersson, M.; Zimmermann, M.B. Subclinical Hypothyroidism and Elevated Thyroglobulin in Infants with Chronic Excess Iodine Intake. Thyroid 2015, 25, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Skeaff, S.A. Thyroglobulin as a biomarker of iodine deficiency: A review. Thyroid 2014, 24, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Venn, B.J.; Manning, P.J.; Cameron, C.M.; Skeaff, S.A. Iodine Supplementation of Mildly Iodine-Deficient Adults Lowers Thyroglobulin: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
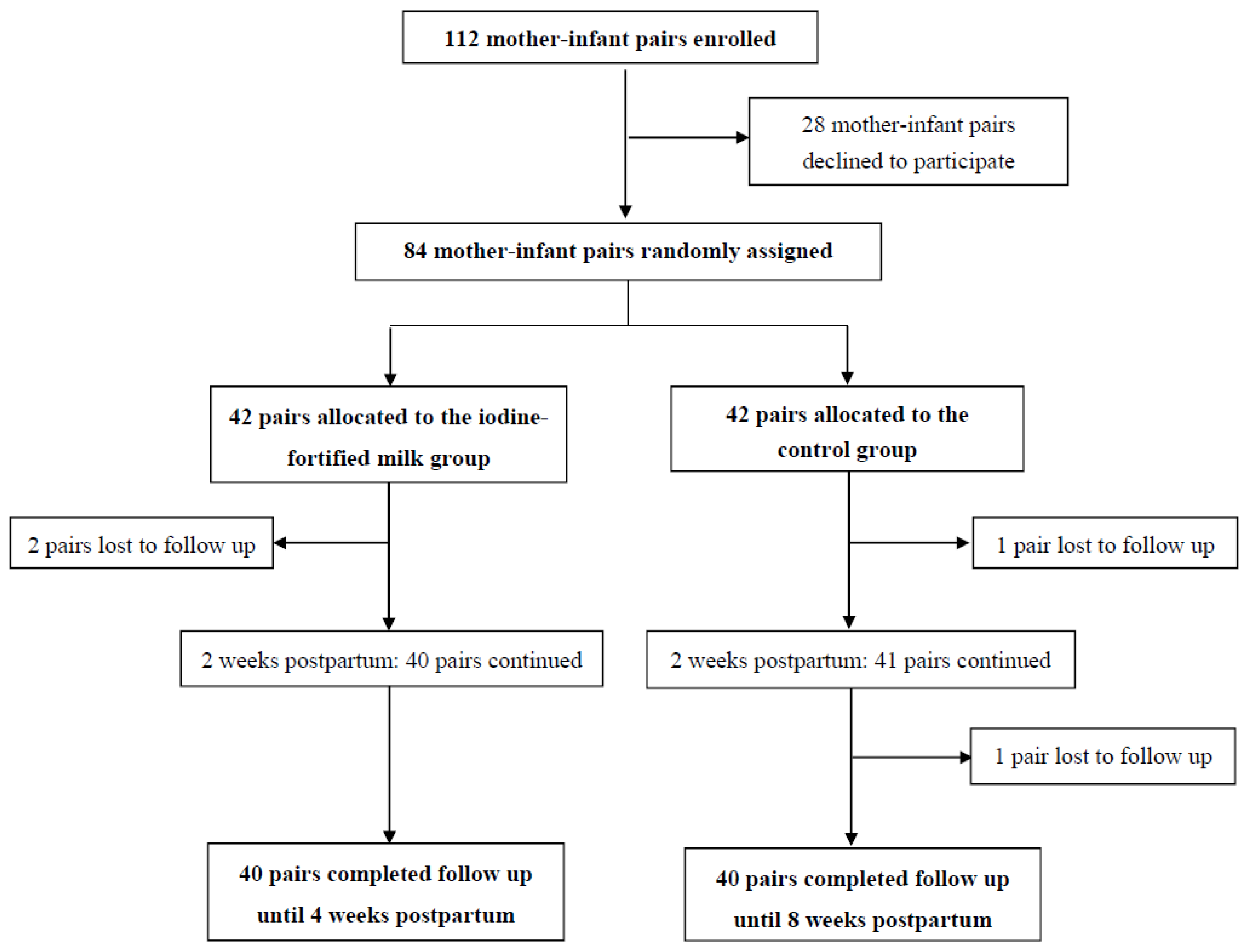
| Characteristics | Iodine Fortified Milk (n = 42) | Control (n = 42) | p |
|---|---|---|---|
| Maternal | |||
| Age (year) | 27.7 ± 4.5 | 28.5 ± 4.5 | 0.356 |
| Education (year) | 10.8 ± 2.6 | 11.2 ± 3.7 | 0.355 |
| Occupation (housekeeper), n (%) | 40 (95.2) | 38 (92.7) | 0.625 |
| Time ofprior pregnancy (year) | 3.1 ± 3.6 | 3.4 ± 3.6 | 0.774 |
| Gravidity, n (%) | 0.524 | ||
| Primigravidity | 17 (40.5) | 11 (26.8) | |
| Multigravidity | 25 (59.5) | 30 (73.2) | |
| Parity, n (%) | 0.546 | ||
| Primiparity | 20 (47.6) | 17 (41.5) | |
| Multiparity | 22 (52.4) | 24 (58.5) | |
| Delivery type, n (%) | 0.690 | ||
| NVD | 14 (33.3) | 12 (29.3) | |
| CS | 28 (66.7) | 29 (70.7) | |
| History of abortion, n (%) | 0.261 | ||
| Yes | 7 (16.7) | 11 (26.8) | |
| No | 35 (83.3) | 30 (73.2) | |
| Use of iodine containing supplements during pregnancy, n (%) | 0.485 | ||
| Yes | 3 (7.1) | 2 (4.9) | |
| No | 29 (69.0) | 33 (80.5) | |
| Do not know | 10 (23.8) | 6 (14.6) | |
| Neonatal | |||
| Sex, n (%) | 0.708 | ||
| Male | 26 (61.9) | 27 (65.9) | |
| Female | 16 (38.1) | 14 (34.1) | |
| Birth weight (g) | 3187 ± 344 | 3251 ± 348 | 0.603 |
| Birth height (cm) | 49.7 ± 1.9 | 50.0 ± 1.7 | 0.657 |
| Birth head circumference (cm) | 34.8 ± 1.2 | 34.5 ± 1.5 | 0.468 |
| TSH (mIU/L) | 1.5 ± 1.7 | 1.2 ± 1.1 | 0.350 |
| Time Postpartum | Iodine Fortified Milk (n = 40) | Control (n = 40) | ||
|---|---|---|---|---|
| Median (IQR) | n (%) * | Median (IQR) | n (%) * | |
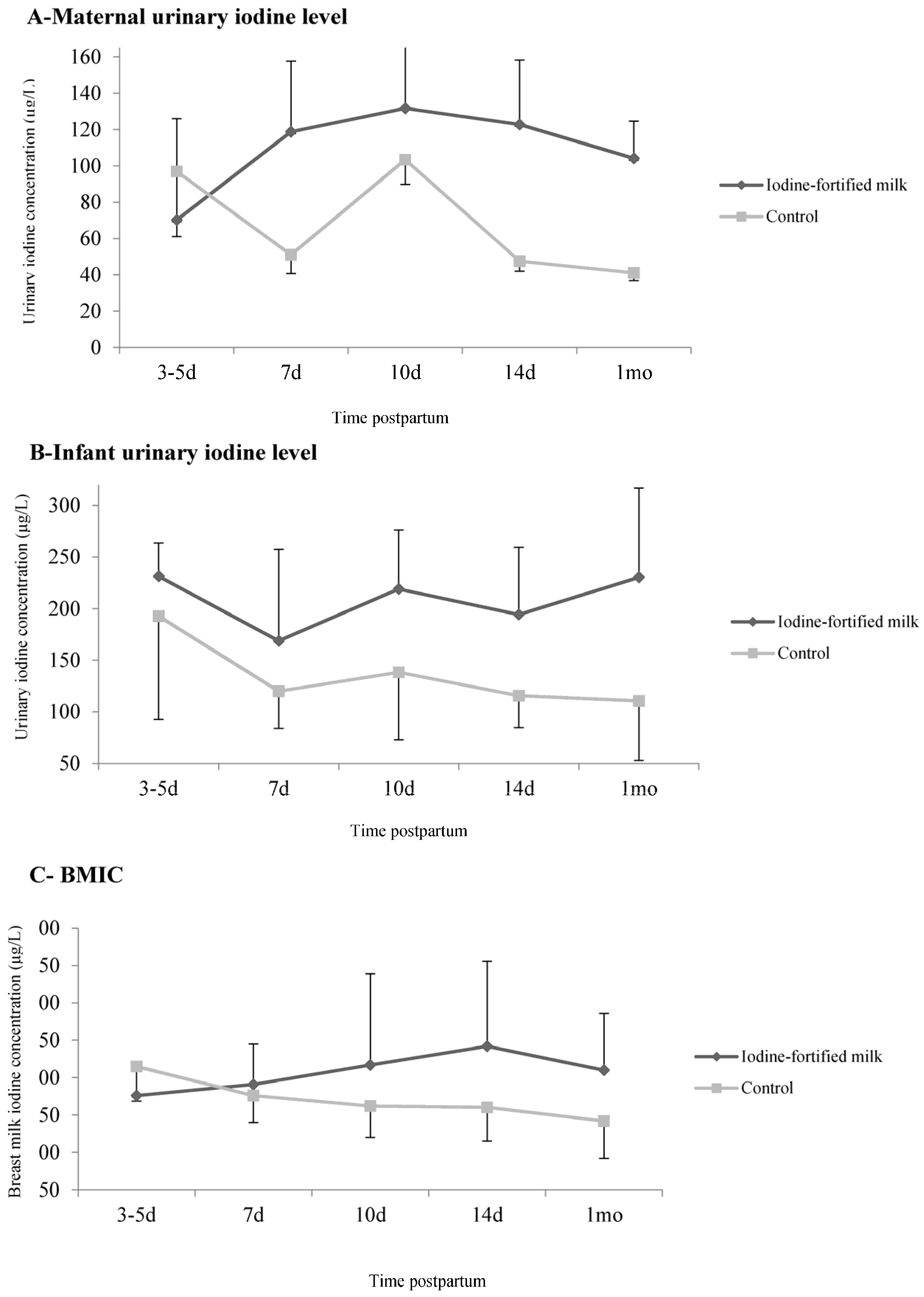
| Maternal UIC (μg/L) | ||||
| 3–5 days (baseline) | 70.2 (41.2–199.0) | 14 (35.9) | 96.9 (47.1–193.8) | 16 (45.7) |
| 7 days | 118.7 (68.0–161.7) ** | 23 (59.0) ** | 51.0 (38.2–77.8) | 6 (15.8) |
| 10 days | 131.7 (85.3–188.4) | 26 (74.3) | 103.4 (88.6–140.7) | 18 (60) |
| 14 days | 122.8 (77.2–182.9) ** | 23 (60.5) ** | 47.5 (38.4–86.8) | 5 (12.2) |
| 1 month | 104.1 (57.3–160.9) | 16 (55.2) | 41.1 (32.1–55.9) | 2 (5.1) |
| BMIC (μg/L) | ||||
| 3–5 days (baseline) | 176.0 (133.7–218.7) ** | 26 (65.0) | 215.0 (168.5–315.5) | 34 (81.0) |
| 7 days | 191.0 (105.0–245.0) | 27 (65.9) | 176.0 (140.0–286.0) | 28 (71.8) |
| 10 days | 217.0 (148.7–339.0) ** | 29 (74.4) | 162.0 (120.0–206.5) | 22 (55.0) |
| 14 days | 242.0 (156.2–355.7) ** | 30 (78.9) | 160.0 (115.2–199.2) | 23 (60.5) |
| 1 month | 210.0 (100.0–286.0) ** | 25 (64.1) | 142.0 (92.2–197.2) | 19 (47.5) |
| Infant UIC (μg/L) | ||||
| 3–5 days (baseline) | 231.2 (91.7–268.2) | 13 (72.2) | 192.8 (79.4–244.6) | 16 (61.5) |
| 7 days | 168.7 (86.2–326.4) | 18 (75.0) | 120.0 (69.7–219.3) | 16 (64.0) |
| 10 days | 218.9 (138.4–293.1) ** | 17 (85.0) | 138.3 (60.0–191.5) | 16 (61.5) |
| 14 days | 194.3 (122.1–306.0) ** | 24 (80.0) ** | 115.7 (75.2–223.3) | 22 (56.4) |
| 1 month | 230.2 (71.0–317.5) ** | 14 (73.7) | 110.4 (47.2–197.0) | 19 (52.8) |
| Variables | Ratio of Means (95% CI) * | p ** |
|---|---|---|
| Maternal urinary iodine concentration | ||
| Model I † | 1.58 (1.53–1.65) | <0.001 |
| Model II ‡ | 1.85 (1.77–1.93) | <0.001 |
| Breast milk iodine concentration | ||
| Model I † | 1.11 (1.07–1.16) | 0.096 |
| Model II ‡ | 1.25 (1.19–1.32) | <0.001 |
| Infant urinary iodine concentration | ||
| Model I § | 1.31 (1.24–1.38) | 0.021 |
| Model II € | 1.19 (0.69–1.45) | 0.400 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazeri, P.; Mirmiran, P.; Tahmasebinejad, Z.; Hedayati, M.; Delshad, H.; Azizi, F. The Effects of Iodine Fortified Milk on the Iodine Status of Lactating Mothers and Infants in an Area with a Successful Salt Iodization Program: A Randomized Controlled Trial. Nutrients 2017, 9, 180. https://doi.org/10.3390/nu9020180
Nazeri P, Mirmiran P, Tahmasebinejad Z, Hedayati M, Delshad H, Azizi F. The Effects of Iodine Fortified Milk on the Iodine Status of Lactating Mothers and Infants in an Area with a Successful Salt Iodization Program: A Randomized Controlled Trial. Nutrients. 2017; 9(2):180. https://doi.org/10.3390/nu9020180
Chicago/Turabian StyleNazeri, Pantea, Parvin Mirmiran, Zhale Tahmasebinejad, Mehdi Hedayati, Hossein Delshad, and Fereidoun Azizi. 2017. "The Effects of Iodine Fortified Milk on the Iodine Status of Lactating Mothers and Infants in an Area with a Successful Salt Iodization Program: A Randomized Controlled Trial" Nutrients 9, no. 2: 180. https://doi.org/10.3390/nu9020180




