The Effects of Moderate Whole Grain Consumption on Fasting Glucose and Lipids, Gastrointestinal Symptoms, and Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
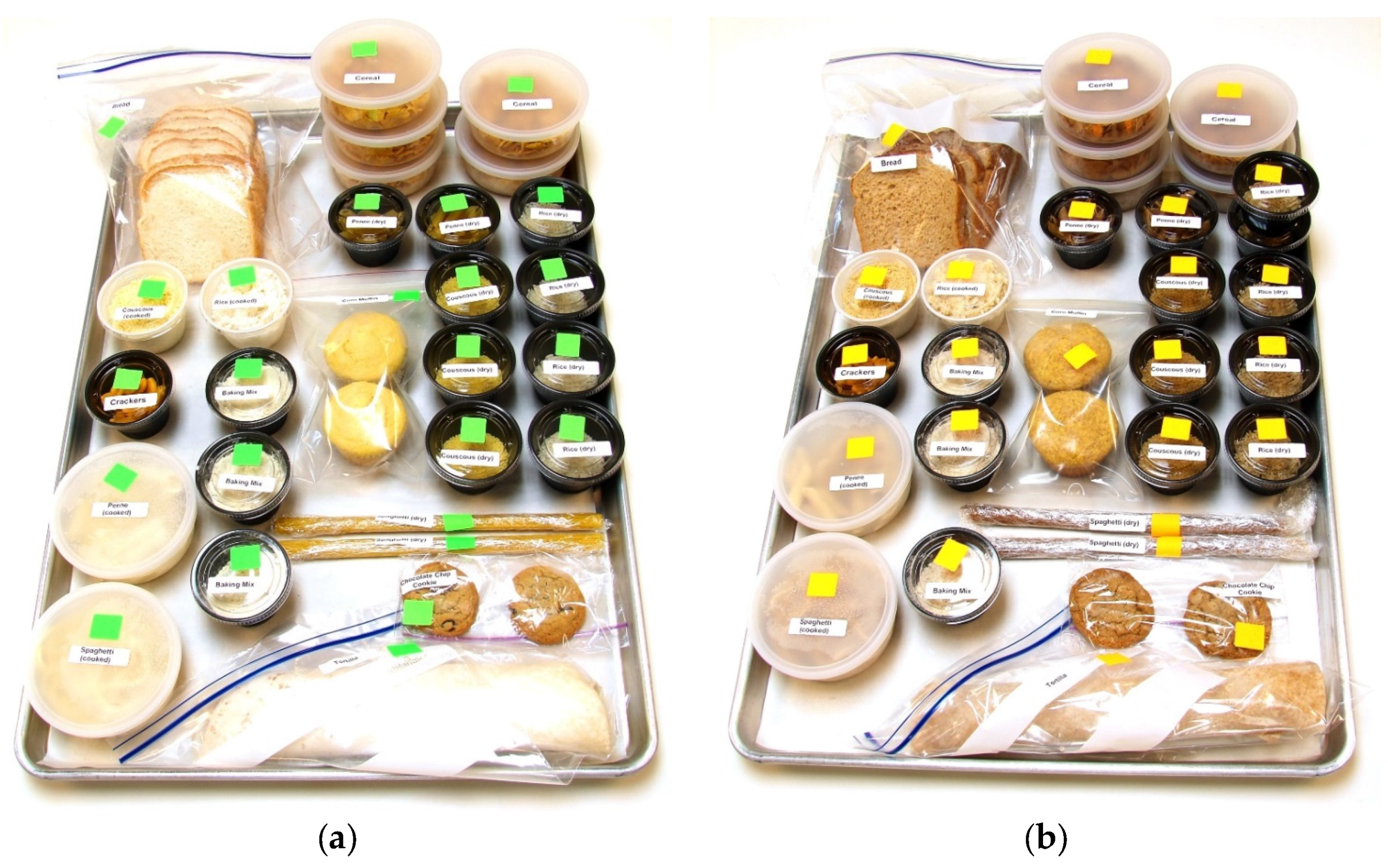
2.2. Market Baskets and Consumption Log
2.3. Body Composition
2.4. Clinical Parameters
2.5. Gastrointestinal Symptoms Log Book
2.6. Fecal Collection
2.7. Gut Microbial Community Analysis
2.8. Statistical Methods
3. Results
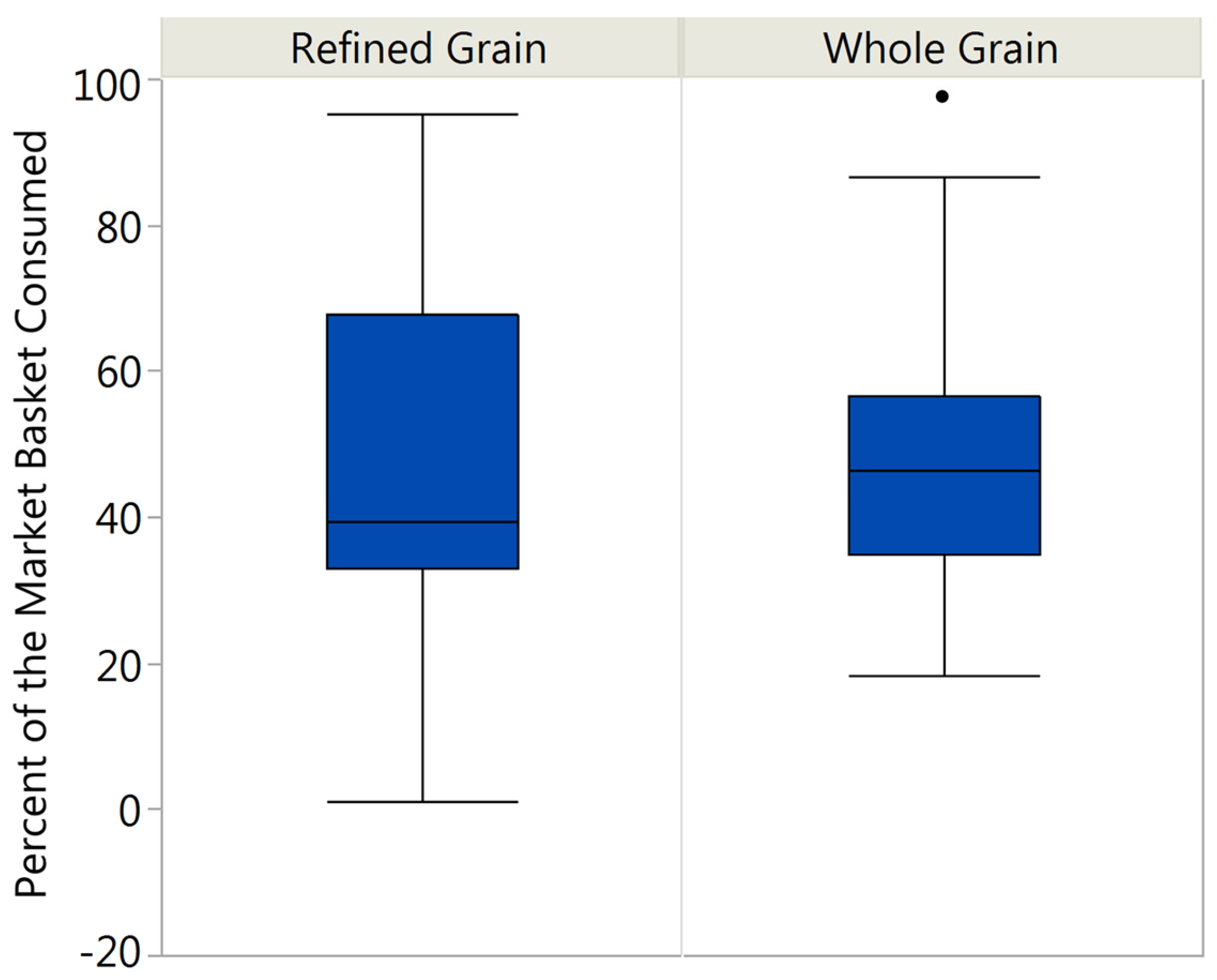
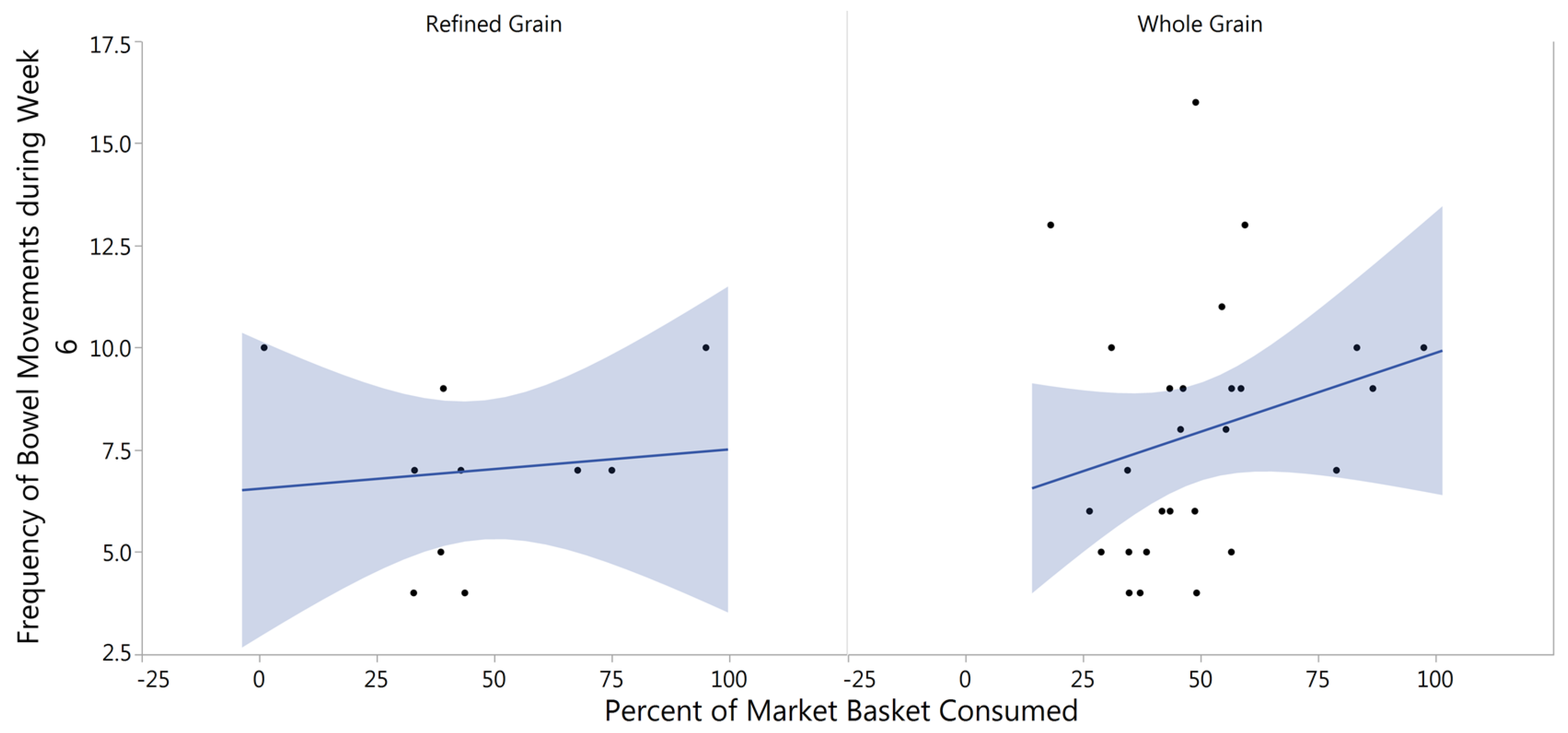
3.1. Market Basket Consumption
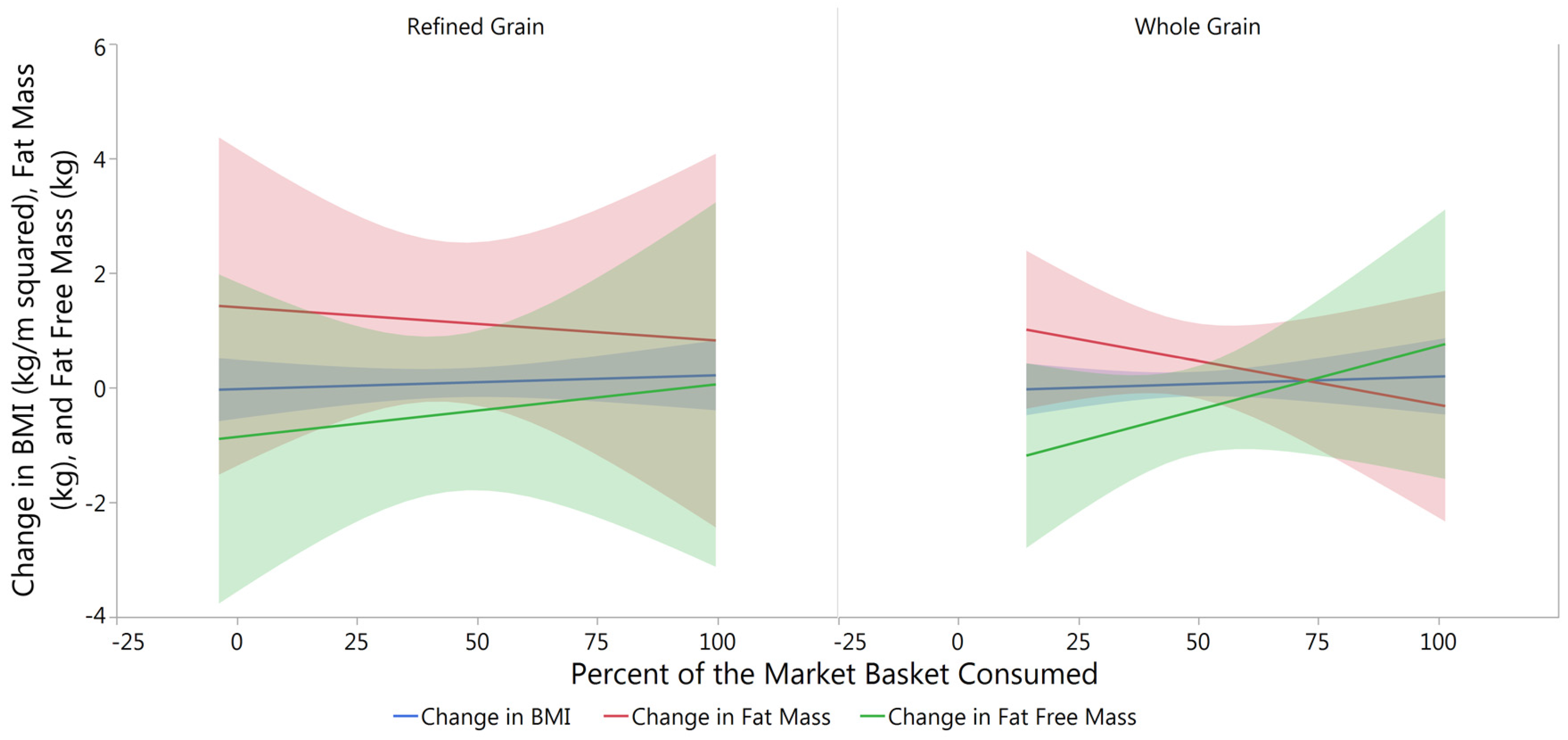
3.2. Changes in Body Composition from Baseline to Post-Intervention
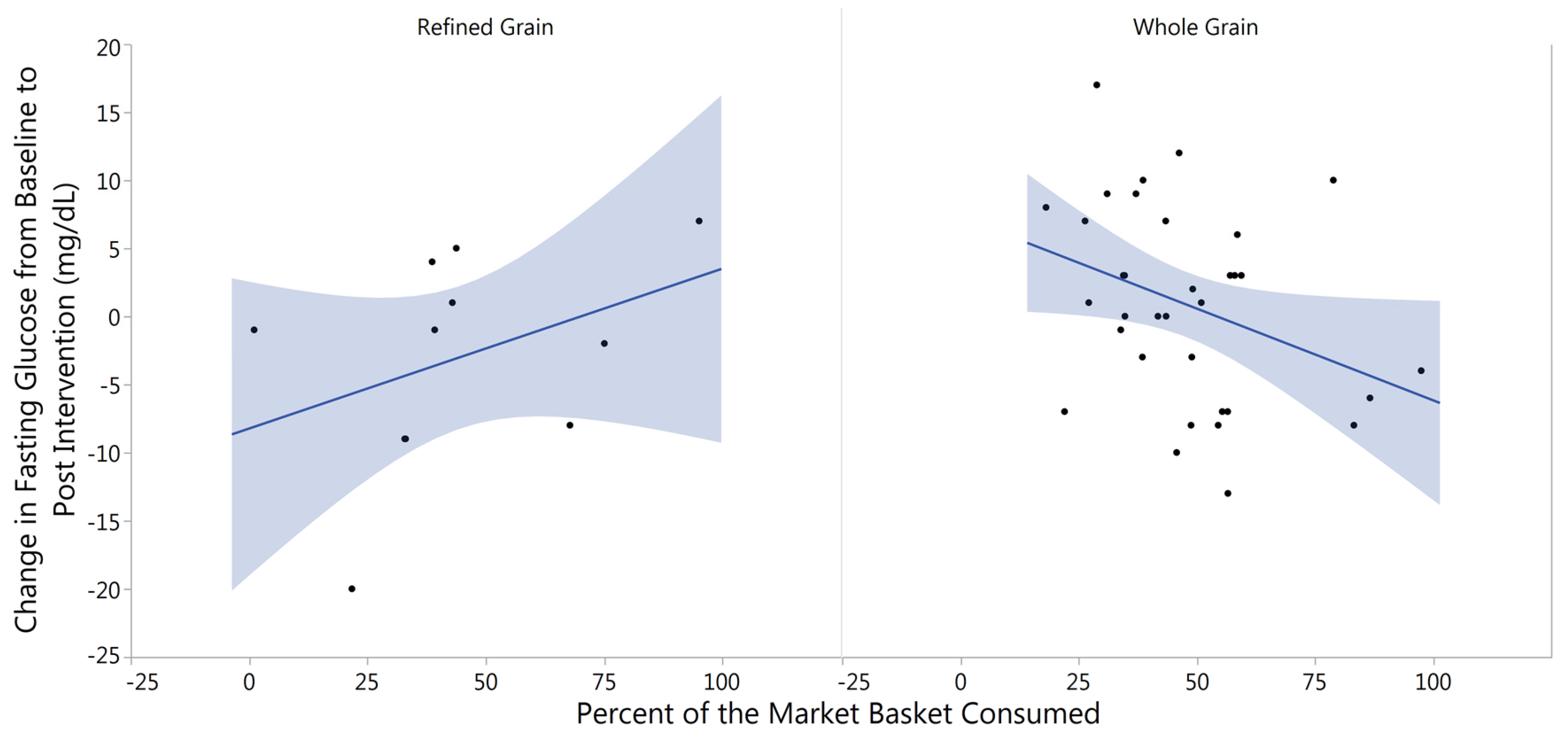
3.3. Changes in Fasting Blood Glucose from Baseline to Post-Intervention
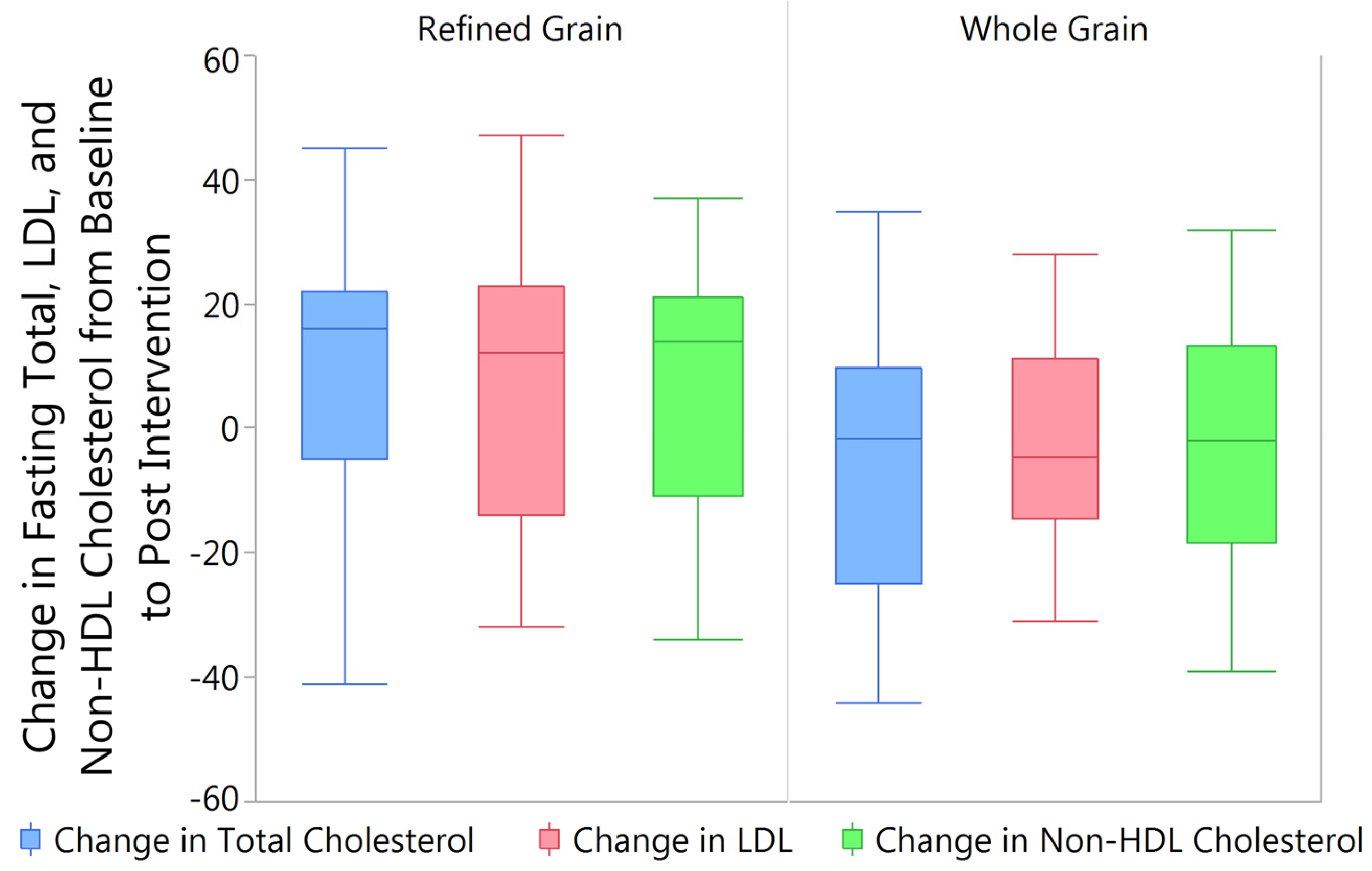
3.4. Changes in Fasting Blood Lipids from Baseline to Post Intervention
3.5. Gastrointestinal Tolerability
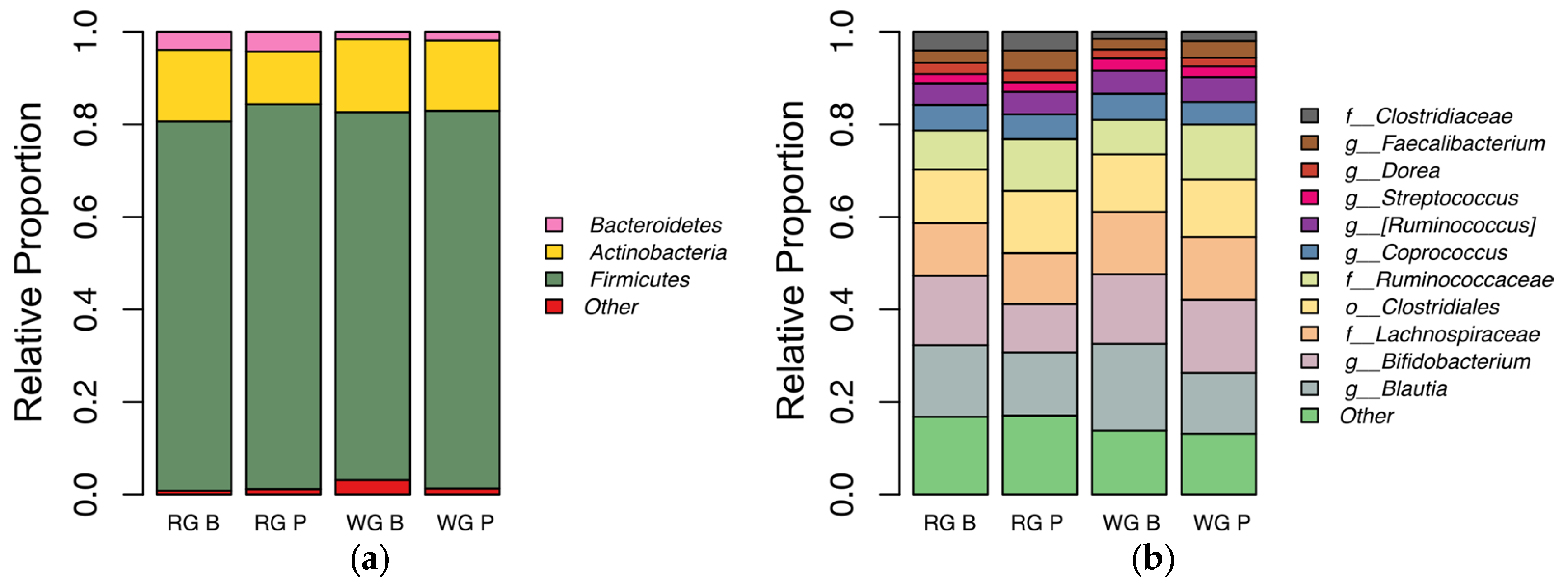
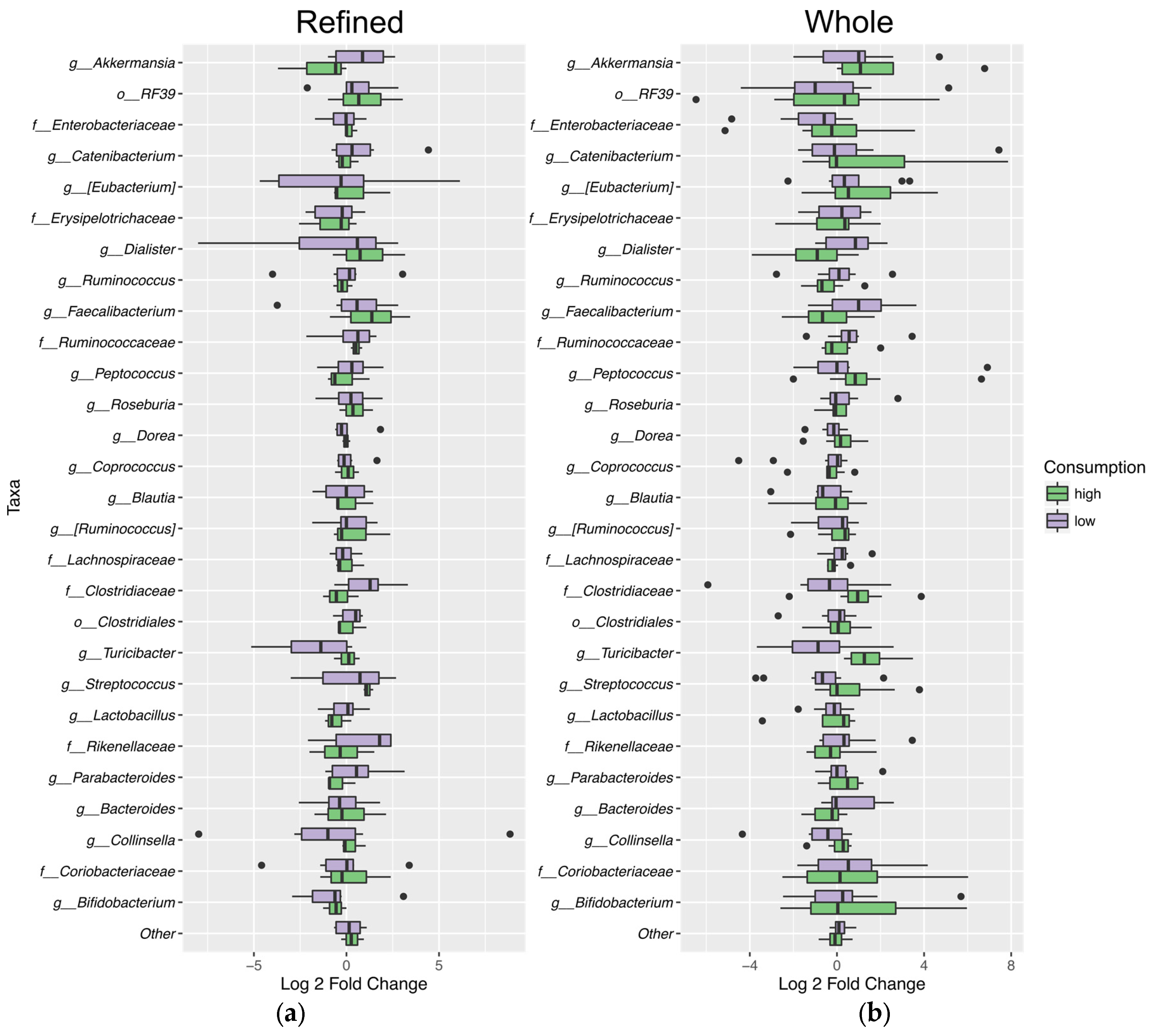
3.6. Fecal Microbiota Analysis
4. Discussion
4.1. Fiber Intake
4.2. Fasting Glucose
4.3. Blood Lipids
4.4. Fecal Frequency
4.5. Microbiota
4.6. Limitations
5. Conclusions
6. Future Research
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Food Availability (Per Capita) Data System. Available online: http://ers.usda.gov/data-products/food-availability-per-capita-data-system.aspx (accessed on 20 April 2016).
- Albertson, A.M.; Good, C.K.; Eldridge, A.L.; Holschuh, N.M. Whole grain consumption and food sources in the united states: Data from nhanes 1999–2000 and the usda’s pyramid servings database for the usda survey food codes. FASEB J. 2005, 19, A87–A87. [Google Scholar]
- McGill, C.; Fulgoni, V.L., III; Devareddy, L. Ten-year trends in fiber and whole grain intakes and food sources for the united states population: National health and nutrition examination survey 2001–2010. Nutrients 2015, 7, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Okarter, N.; Liu, R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Van der Kamp, J.W.; Poutanen, K.; Seal, C.J.; Richardson, D.P. The healthgrain definition of ’whole grain’. Food Nutr. Res. 2014, 58, 22100. [Google Scholar] [CrossRef] [PubMed]
- International, AACC. Whole grain definition. Cereal Foods World 1999, 45, 79. [Google Scholar]
- Bett-Garber, K.L.; Lea, J.M.; Champagne, E.T.; McClung, A.M. Whole-grain rice flavor associated with assorted bran colors. J. Sens. Stud. 2012, 27, 78–86. [Google Scholar] [CrossRef]
- Slavin, J. Whole grains and digestive health. Cereal Chem. 2010, 87, 292–296. [Google Scholar] [CrossRef]
- Slavin, J.L. Whole grains, refined grains and fortified refined grains: What’s the difference? Asia Pac. J. Clin. Nutr. 2000, 9, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Marquart, L.; Chan, H.W.; Orsted, M.; Schmitz, K.A.; Arndt, E.A.; Jacobs, D.R. Gradual incorporation of whole-grain flour into grain-based products. Cereal Foods World 2006, 51, 114–117. [Google Scholar] [CrossRef]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Aromaa, A.; Reunanen, A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 622–629. [Google Scholar] [PubMed]
- Borneo, R.; Leon, A.E. Whole grain cereals: Functional components and health benefits. Food Funct. 2012, 3, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ampatzoglou, A.; Atwal, K.K.; Maidens, C.M.; Williams, C.L.; Ross, A.B.; Thielecke, F.; Jonnalagadda, S.S.; Kennedy, O.B.; Yaqoob, P. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J. Nutr. 2015, 145, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.N.; Martin, R.J.; Keim, N.L. Does whole grain consumption alter gut microbiota and satiety? Healthcare 2015, 3, 364–392. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.H.; West, S.G.; Vanden Heuvel, J.P.; Jonnalagadda, S.S.; Ross, A.B.; Hill, A.M.; Grieger, J.A.; Lemieux, S.K.; Kris-Etherton, P.M. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: A randomized controlled-feeding trial. Am. J. Clin. Nutr. 2014, 100, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Modi, P. Benefits of low glycemic and high satiety index foods for obesity and diabetes control and management. In Nutrients, Dietary Supplements, and Nutriceuticals; Cost Analysis Versus Clinical Benefits; Springer: New York, NY, USA, 2011; pp. 403–424. [Google Scholar]
- Zhou, A.L.; Hergert, N.; Rompato, G.; Lefevre, M. Whole grain oats improve insulin sensitivity and plasma cholesterol profile and modify gut microbiota composition in C57BL/6J mice. J. Nutr. 2015, 145, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. Whole grains and pulses: A comparison of the nutritional and health benefits. J. Agric. Food Chem. 2014, 62, 7029–7049. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Foglian, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Raninen, K.; Lappi, J.; Mykkanen, H.; Poutanen, K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nyman, M.; Siljestrom, M.; Pedersen, B.; Knudsen, K.E.B.; Asp, N.G.; Johansson, C.G.; Eggum, B.O. Dietary fiber content and composition in 6 cereals at different extraction rates. Cereal Chem. 1984, 61, 14–19. [Google Scholar]
- Frankenfield, D.C.; Muth, E.R.; Rowe, W.A. The harris-benedict studies of human basal metabolism: History and limitations. J. Am. Diet. Assoc. 1998, 98, 439–445. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.W. A study of the likert technique of attitude scale construction. J. Soc. Psychol. 1941, 13, 51–57. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Peterson, C.M.; Raggio, A.; Keenan, M.J.; Martin, R.J.; Ravussin, E.; Marco, M.L. Impact of different fecal processing methods on assessments of bacterial diversity in the human intestine. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Nikkila, J.; Jalanka-Tuovinen, J.; Immonen, O.; Rajilic-Stojanovic, M.; Kekkonen, R.A.; Palva, A.; de Vos, W.M. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: Effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J. Microbiol. Methods 2010, 81, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive bayesian classifier for rapid assignment of rrna sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Hamady, M.; Walker, J.J.; Harris, J.K.; Gold, N.J.; Knight, R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 2008, 5, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Aronesty, E. Ea-utils: Command-Line Tools for Processing Biological Sequencing Data. Available online: https://github.com/ExpressionAnalysis/ea-utils (accessed on 17 April 2011).
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Lewis, J.D.; Hoffmann, C.; Chen, Y.Y.; Knight, R.; Bittinger, K.; Hwang, J.; Chen, J.; Berkowsky, R.; Nessel, L.; et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16s sequence tags. BMC Microbiol. 2010, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.S.; Holland, B.; Cheung, S.H. A modified benjamini-hochberg multiple comparisons procedure for controlling the false discovery rate. J. Stat. Plan. Inference 2002, 104, 351–362. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from europe and rural africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.D.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Lange, E. Oats products as functional food. Zywnosc-Nauka Technol. Jakosc 2010, 17, 7–24. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Bressani, R.; Breuner, M.; Ortiz, M.A. Acid-detergent and neutro-detergent fiber and mineral elements content of maize and tortilla. Arch. Latinoam. Nutr. 1989, 39, 382–391. [Google Scholar] [PubMed]
- Sumczynski, D.; Bubelova, Z.; Fisera, M. Determination of chemical, insoluble dietary fibre, neutral-detergent fibre and in vitro digestibility in rice types commercialized in Czech markets. J. Food Compos. Anal. 2015, 40, 8–13. [Google Scholar] [CrossRef]
- Lai, V.M.F.; Lu, S.; He, W.H.; Chen, H.H. Non-starch polysaccharide compositions of rice grains with respect to rice variety and degree of milling. Food Chem. 2007, 101, 1205–1210. [Google Scholar] [CrossRef]
- Rose, D.J.; Patterson, J.A.; Hamaker, B.R. Structural differences among alkali-soluble arabinoxylans from maize (Zea mays), rice (oryza sativa), and wheat (triticum aestivum) brans influence human fecal fermentation profiles. J. Agric. Food Chem. 2010, 58, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Spiegelman, D.; Sudha, V.; Gayathri, R.; Hong, B.; Praseena, K.; Anjana, R.M.; Wedick, N.M.; Arumugam, K.; Malik, V.; et al. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: A randomized controlled trial. Diabetes Technol. Ther. 2014, 16, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ranawana, V.; Clegg, M.E.; Shafat, A.; Henry, C.J. Postmastication digestion factors influence glycemic variability in humans. Nutr. Res. 2011, 31, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, G.M.; Stroebinger, N.; Rutherfurd, S.M.; Singh, R.P.; Moughan, P.J. Properties of gastric chyme from pigs fed cooked brown or white rice. Food Biophys. 2013, 8, 12–23. [Google Scholar] [CrossRef]
- Oishi, K.; Yamamoto, S.; Itoh, N.; Nakao, R.; Yasumoto, Y.; Tanaka, K.; Kikuchi, Y.; Fukudome, S.; Okita, K.; Takano-Ishikawa, Y. Wheat alkylresorcinols suppress high-fat, high-sucrose diet-induced obesity and glucose intolerance by increasing insulin sensitivity and cholesterol excretion in male mice. J. Nutr. 2015, 145, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Sagen, E.K.; Varghese, Z.; MacDougall, K.; Preston, T. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adult. BMJ 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D.J.A. Propionate lowers blood-glucose and alters lipid-metabolism in healthy-subjects. Am. J. Clin. Nutr. 1991, 54, 860–865. [Google Scholar] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.S.; Vorster, H.H.; Cummings, J.H. Effects of dietary propionate on carbohydrate and lipid-metabolism in healthy-volunteers. Am. J. Gastroenterol. 1990, 85, 549–553. [Google Scholar] [PubMed]
- Johansson, E.V.; Nilsson, A.C.; Ostman, E.M.; Bjorck, I.M.E. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr. J. 2013, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.H.; Noakes, M.; Royle, P.J.; Foster, P.R. Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men. Am. J. Clin. Nutr. 2003, 77, 967–974. [Google Scholar] [PubMed]
- Youn, M.; Csallany, A.S.; Gallaher, D.D. Whole grain consumption has a modest effect on the development of diabetes in the goto-kakisaki rat. Br. J. Nutr. 2012, 107, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Jensen, M.G.; Riboldi, G.; Petronio, M.; Bugel, S.; Toubro, S.; Tetens, I.; Astrup, A. Wholegrain vs. Refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite 2010, 54, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Cano, J.M.M.; Aguilar, A.C.; Hernandez, J.C. Lipid-lowering effect of maize-based traditional mexican food on a metabolic syndrome model in rats. Lipids Health Dis. 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Bajka, B.; Rigby, N. Roles for dietary fibre in the upper gi tract: The importance of viscosity. Food Res. Int. 2016, 88, 234–238. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Laerke, H.N. Whole grain cereals and gut health. Agro Food Ind. Hi-Tech 2008, 19, 6–8. [Google Scholar]
- Odes, H.S.; Lazovski, H.; Stern, I.; Madar, Z. Double-blind trial of a high dietary fiber, mixed grain cereal in patients with chronic constipation and hyperlipidemia. Nutr. Res. 1993, 13, 979–985. [Google Scholar] [CrossRef]
- Bird, A.R.; Vuaran, M.S.; King, R.A.; Noakes, M.; Keogh, J.; Morell, M.K.; Topping, D.L. Wholegrain foods made from a novel high-amylose barley variety (himalaya 292) improve indices of bowel health in human subjects. Br. J. Nutr. 2008, 99, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Son, B.K.; Lee, S.S. Effects of adlay, buckwheat, and barley on transit time and the antioxidative system in obesity induced rats. Nutr. Res. Pract. 2012, 6, 208–212. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Miller, P.E.; Verbeke, K. Effects of cereal fiber on bowel function: A systematic review of intervention trials. World J. Gastroenterol. 2015, 21, 8952–8963. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Birkett, A.; Hulshof, T.; Verbeke, K.; Gibes, K. Effects of cereal, fruit and vegetable fibers on human fecal weight and transit time: A comprehensive review of intervention trials. Nutrients 2016, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Health aspects of dietary fibre. Fibre-Rich Wholegrain Foods Improv. Qual. 2013, 237, 61–75. [Google Scholar]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Toward a road map for global -omics: A primer on -omic technologies. Am. J. Epidemiol. 2014, 180, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. Fems Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Mirande, C.; Kadlecikova, E.; Matulova, M.; Capek, P.; Bernalier-Donadille, A.; Forano, E.; Bera-Maillet, C. Dietary fibre degradation and fermentation by two xylanolytic bacteria bacteroides xylanisolvens xb1at and roseburia intestinalis xb6b4 from the human intestine. J. Appl. Microbiol. 2010, 109, 451–460. [Google Scholar] [PubMed]
- Hamer, H.M.; De Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Bosscher, D.; Breynaert, A.; Pieters, L.; Hermans, N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J. Physiol. Pharmacol. 2009, 60, 5–11. [Google Scholar] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.G.; Licht, T.R.; Kristensen, M.; Bahl, M.I. Bifidogenic effect of whole-grain wheat during a 12-week energy-restricted dietary intervention in postmenopausal women. Eur. J. Clin. Nutr. 2013, 67, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Backhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Mobley, A.R.; Jones, J.M.; Rodriguez, J.; Slavin, J.; Zelman, K.M. Identifying practical solutions to meet america’s fiber needs: Proceedings from the food & fiber summit. Nutrients 2014, 6, 2540–2551. [Google Scholar] [PubMed]
- Zhong, Y.; Nyman, M.; Fak, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Gahan, C.G.M. The gut microbiota and the metabolic health of the host. Curr. Opin. Gastroenterol. 2014, 30, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.L.; Dong, X.X.; Moraes, A.C.F.; Zielke, R.A.; Fernandes, G.R.; Peremyslova, E.; Vasquez-Perez, S.; Schoenborn, A.A.; Gomes, E.P.; Pereira, A.C.; et al. Akkermansia muciniphila mediates negative effects of ifn gamma on glucose metabolism. Nat. Commun. 2016, 7, 13329. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Tesar, I.; Hippe, B.; Gnauer, S.; Rust, P.; Haslberger, A.G. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 2015, 6, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Hang, I.; Rinttila, T.; Zentek, J.; Kettunen, A.; Alaja, S.; Apajalahti, J.; Harmoinen, J.; de Vos, W.M.; Spillmann, T. Effect of high contents of dietary animal-derived protein or carbohydrates on canine faecal microbiota. BMC Vet. Res. 2012, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Berry, D.; Rauch, I.; Rennisch, I.; Ramesmayer, J.; Hainzl, E.; Heider, S.; Decker, T.; Kenner, L.; Muller, M.; et al. Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. 2014, 8, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.Y.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Garcia-Carpintero, S.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Delgado-Lista, J.; Perez-Martinez, P.; Zuniga, O.A.R.; Quintana-Navarro, G.M.; Landa, B.B.; Clemente, J.C.; et al. The gut microbial community in metabolic syndrome patients is modified by diet. J. Nutr. Biochem. 2016, 27, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Liu, F.L.; Ling, Z.X.; Tong, X.J.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.M.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.Y.; Petrosino, J.F.; Correa, A.; He, J. Gut microbiome associates with lifetime cardiovascular disease risk profile among bogalusa heart study participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the role of erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
| Screening | Baseline Test Day | Six Week Intervention (Each Event Occurred Weekly) | Post-Intervention Test Day |
|---|---|---|---|
|
|
|
|
| Subjects | Sex | Age (Years) | BMI (kg/m2) | Calculated Daily Calorie Needs (kcal/day) | Percent of Market Basket Consumed |
|---|---|---|---|---|---|
| F = Female | |||||
| M = Male | |||||
| All Subjects in Sample | |||||
| Total (n = 46) | 25 F, 21 M | 25.8 ± 0.9 | 23.4 ± 0.6 | 2247.8 ± 48.2 | 47.1 ± 2.9 |
| RG (n = 11) | 3 F, 8 M | 24.6 ± 1.6 | 25.5 ± 2.1 | 2363.6 ± 96.6 | 44.7 ± 7.8 |
| WG (n = 35) | 22 F, 13 M | 26.2 ± 1 | 22.8 ± 0.5 | 2211.4 ± 55 | 47.9 ± 3 |
| Consumable | Refined Grain Products | Whole Grain Products | Servings/Week | ||
|---|---|---|---|---|---|
| Food Item | Description | Brand | Description | Brand | At 2000 kcal |
| Wheat bread | White, slices | Sysco Classic | 100% whole wheat, slices | Hi Vibe | 7 slices |
| Cereal | Cornflakes | Kellogg’s | Wheaties | General Mills | 5 cups |
| Cookie | Chocolate chip with white enriched flour | Recipe developed for study | Chocolate chip with whole wheat flour | Recipe developed for study | 2 cookies, 2 ½ inch diameter |
| Couscous | Refined Wheat | Giusto | Whole Wheat | Woodland Farms | ½ cup prepared + 3 oz. dry |
| Crackers | Goldfish, cheddar, original | Pepperidge Farms | Goldfish, cheddar, whole wheat | Pepperidge Farms | 26 crackers |
| Corn Muffin | Made with finely ground cornmeal | Recipe developed for study | Made with whole kernel cornmeal | Recipe developed for study | 2 muffins |
| Penne Pasta | Semolina Wheat | La Bella | Semolina Whole Wheat | La Bella | ½ cup prepared + 2 oz. dry |
| Rice | Long-rain, white | Sysco Classic | Long-grain, brown | Sysco Classic | ½ cup prepared + 4 oz. dry |
| Spaghetti | Semolina Wheat | La Bella | Semolina, Whole Wheat | La Bella | ½ cup prepared + 2 oz. dry |
| Tortilla | Wheat | Mi Rancho | Whole Wheat | Mi Rancho | 1 tortilla (12-inch diameter) |
| Baking Mix | Based on enriched white flour | Formula developed for study | Based on whole wheat flour | Formula developed for study | 1 cup |
| Treatment | Change in BMI (kg/m2) | Change in Fat Mass (kg) | Change in Fat Free Mass (kg) |
|---|---|---|---|
| RG | 0.01 ± 0.11 | 1.13 ± 0.57 | −0.45 ± 0.56 |
| WG | 0.05 ± 0.10 | 0.49 ± 0.31 | −0.43 ± 0.37 |
| p value | 0.846 | 0.936 | 0.936 |
| Treatment | Bloating and Gas | Abdominal Pain | Nausea | Flatulence | Change in Stool | Feeling about Change in Stool | Bowel Movement Frequency | Average Bristol Score |
|---|---|---|---|---|---|---|---|---|
| Week 1 Responses | ||||||||
| RG | 0.80 ± 0.29 | 0.70 ± 0.30 | 0.60 ± 0.31 | 0.90 ± 0.38 | 0.8 ± 0.25 | 2.14 ± 0.25 | 7.00 ± 0.61 | 3.18 ± 0.23 |
| WG | 0.78 ± 0.20 | 0.56 ± 0.12 | 0.38 ± 0.12 | 1.04 ± 0.24 | 1.17 ± 0.21 | 2.14 ± 0.23 | 8.28 ± 0.65 | 4.16 ± 0.54 |
| p value | 0.824 | 0.863 | 0.595 | 0.573 | 0.371 | 0.785 | 0.397 | 0.088 |
| Week 6 Responses | ||||||||
| RG | 0.40 ± 0.22 | 0.20 ± 0.13 | 0.10 ± 0.10 | 0.60 ± 0.31 | 0.90 ± 0.28 | 2.00± 0.19 | 7.00 ± 0.70 | 3.42 ± 0.25 |
| WG | 0.64 ± 0.14 | 0.44 ± 0.10 | 0.40 ± 0.14 | 0.68 ± 0.18 | 0.92 ± 0.20 | 2.18 ± 0.23 | 7.93 ± 0.59 | 3.41 ± 0.14 |
| p value | 0.307 | 0.198 | 0.187 | 0.774 | 0.952 | 0.518 | 0.535 | 0.959 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, D.N.; Kable, M.E.; Marco, M.L.; De Leon, A.; Rust, B.; Baker, J.E.; Horn, W.; Burnett, D.; Keim, N.L. The Effects of Moderate Whole Grain Consumption on Fasting Glucose and Lipids, Gastrointestinal Symptoms, and Microbiota. Nutrients 2017, 9, 173. https://doi.org/10.3390/nu9020173
Cooper DN, Kable ME, Marco ML, De Leon A, Rust B, Baker JE, Horn W, Burnett D, Keim NL. The Effects of Moderate Whole Grain Consumption on Fasting Glucose and Lipids, Gastrointestinal Symptoms, and Microbiota. Nutrients. 2017; 9(2):173. https://doi.org/10.3390/nu9020173
Chicago/Turabian StyleCooper, Danielle N., Mary E. Kable, Maria L. Marco, Angela De Leon, Bret Rust, Julita E. Baker, William Horn, Dustin Burnett, and Nancy L. Keim. 2017. "The Effects of Moderate Whole Grain Consumption on Fasting Glucose and Lipids, Gastrointestinal Symptoms, and Microbiota" Nutrients 9, no. 2: 173. https://doi.org/10.3390/nu9020173




