Chemopreventive Potential of Raw and Roasted Pistachios Regarding Colon Carcinogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Roasting
2.2. In Vitro Fermentation
2.3. Cell Culture
2.4. DAPI Assay
2.5. Comet Assay
2.6. Isolation of Total RNA
2.7. cDNA Synthesis and RT-qPCR
2.8. Flow Cytometry and Caspase Assay
2.9. Statistical Analyses
3. Results
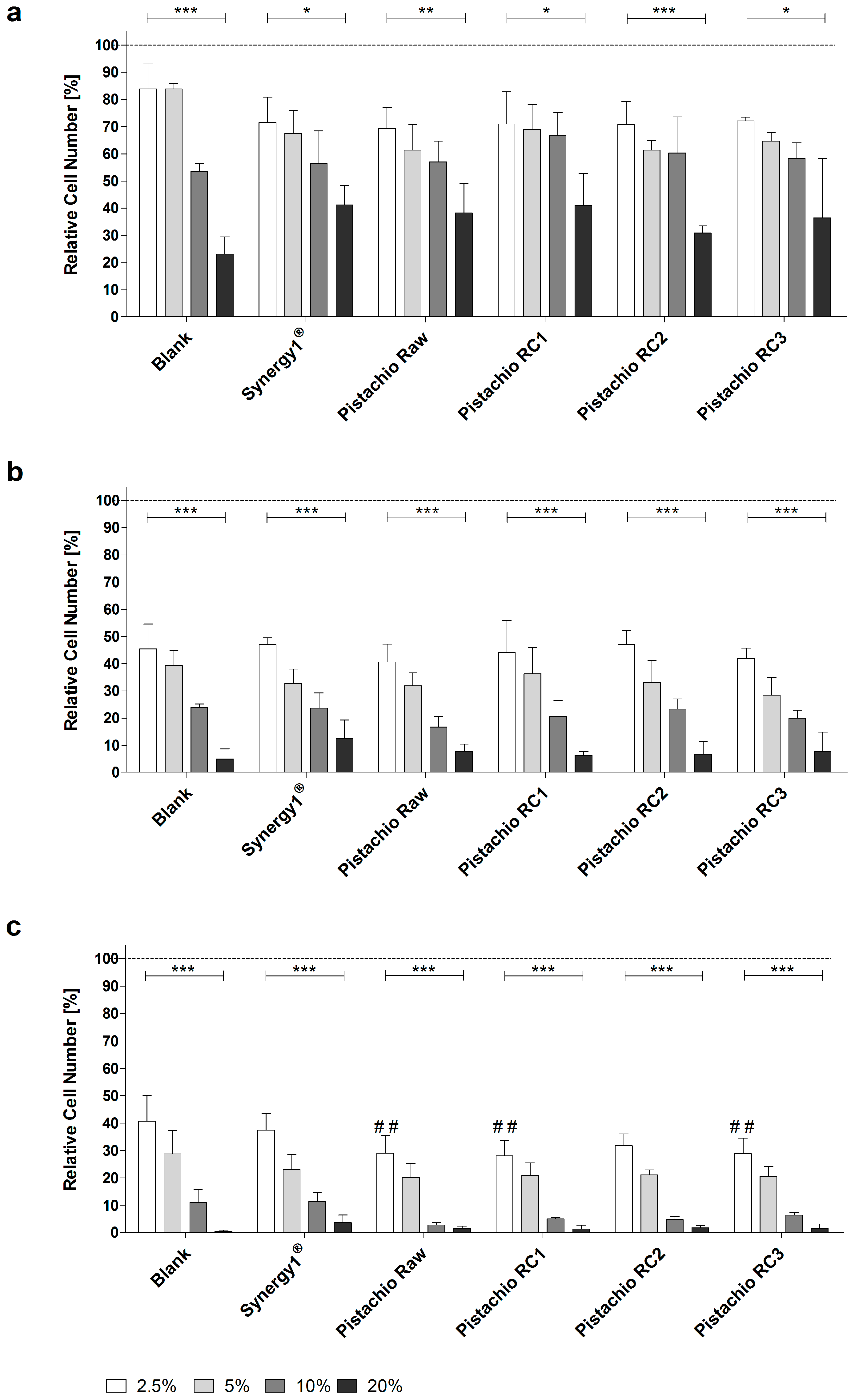
3.1. Pistachio FS Induce Growth Inhibition
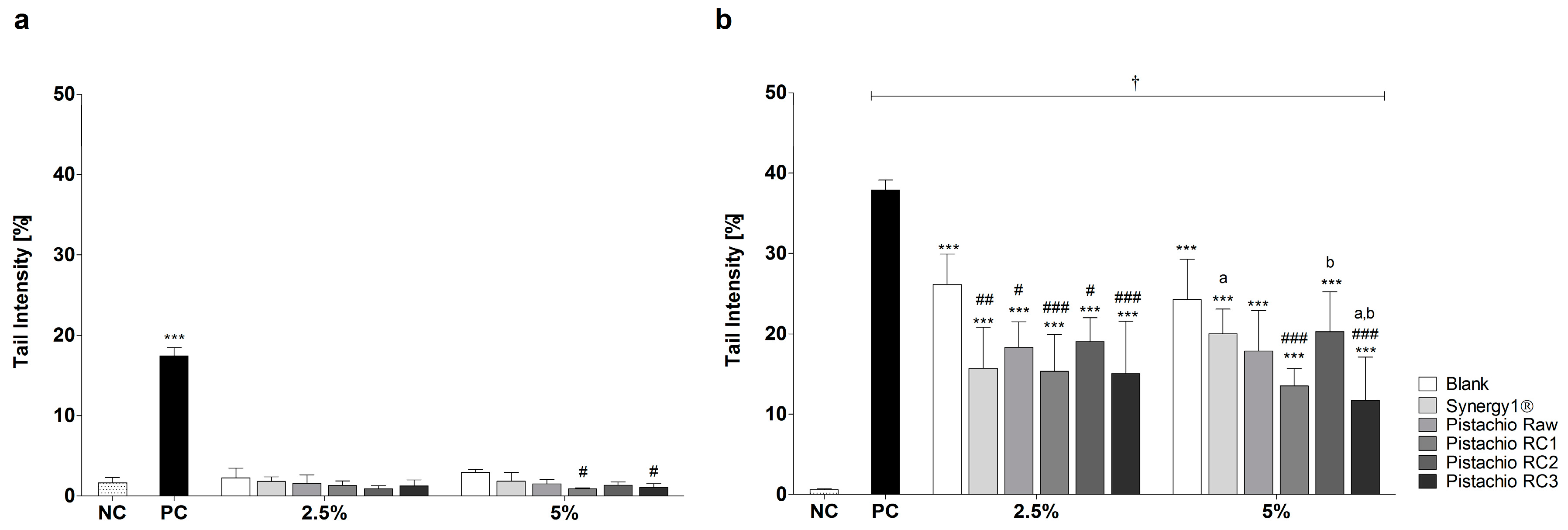
3.2. Pistachio FS Reduce H2O2-Induced DNA Damage
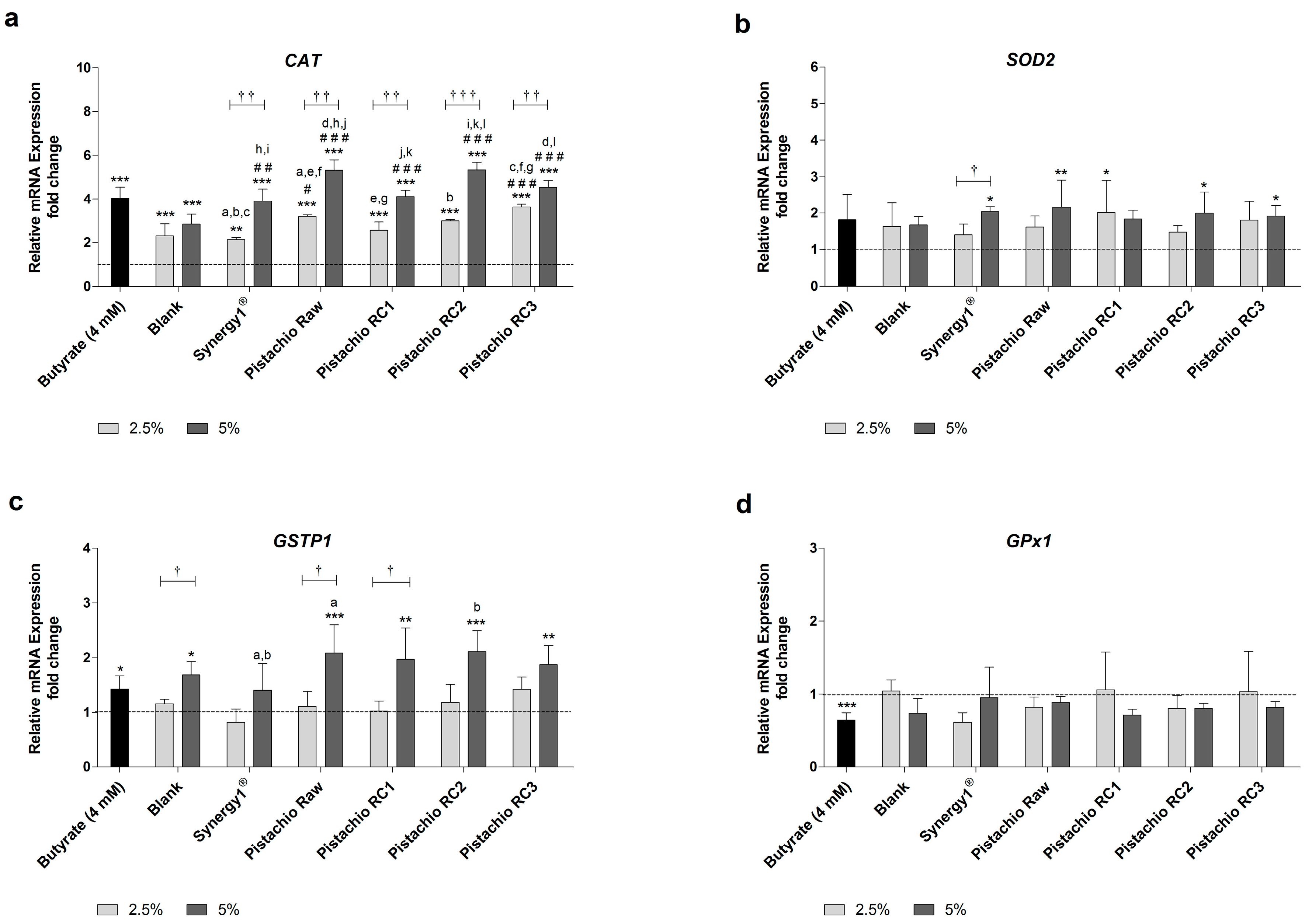
3.3. Pistachio FS Modulate Gene Expression of CAT, SOD2 and GSTP1
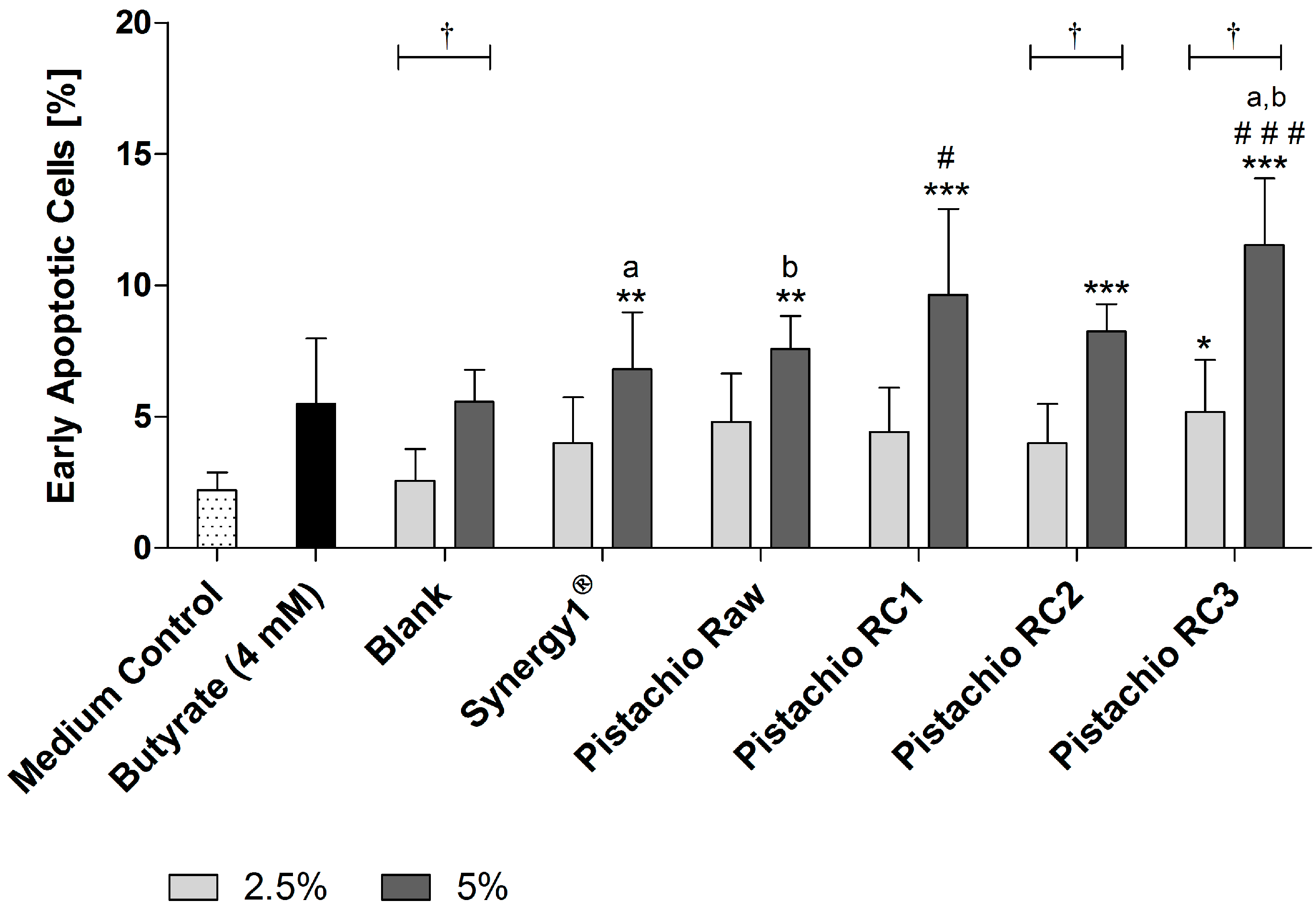
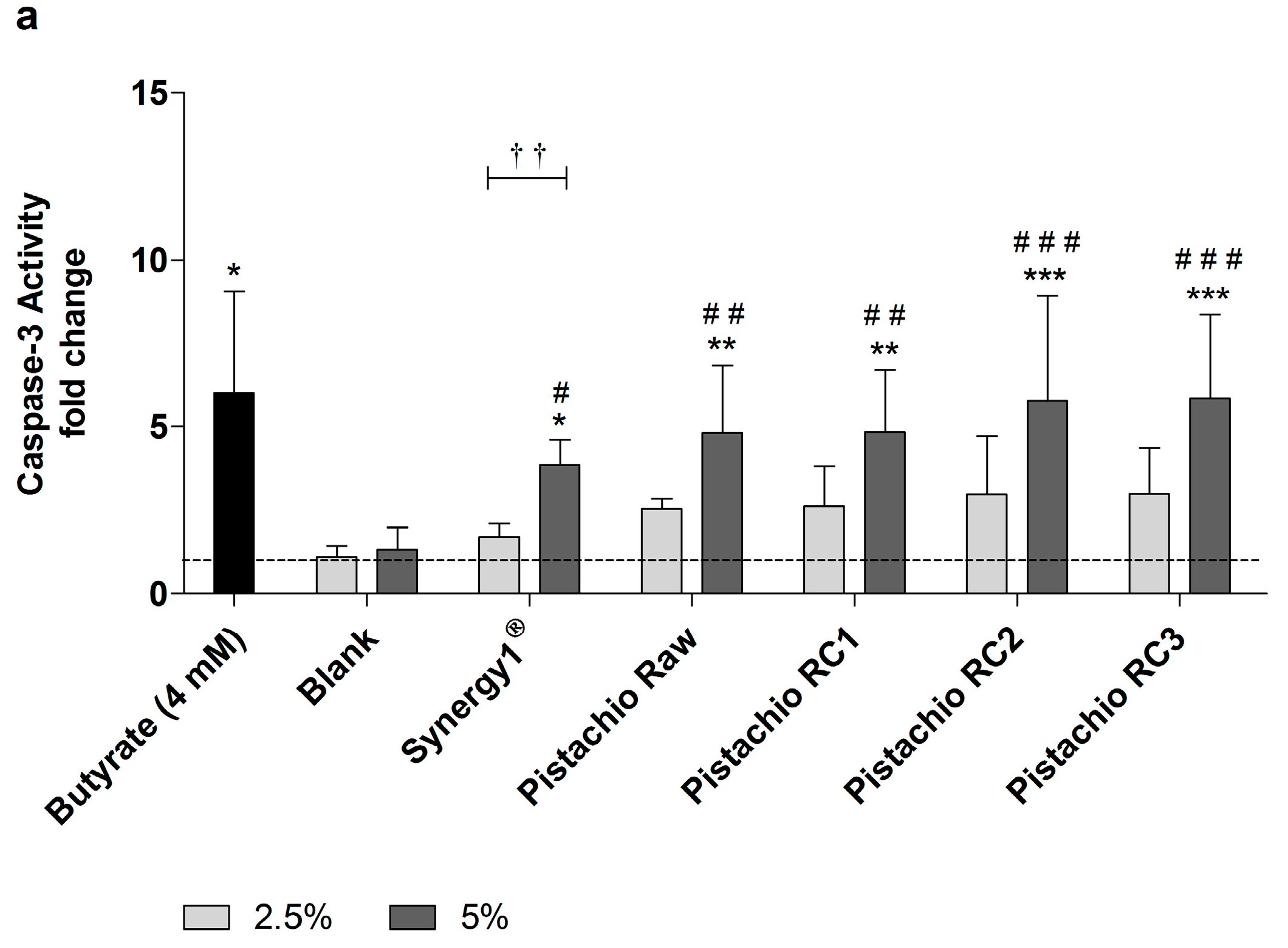
3.4. Pistachio FS Induce Apoptosis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fischer, S.; Glei, M. Health-Potential of Nuts. Ernaehrungs Umsch. Int. 2013, 60, 206–215. [Google Scholar]
- Wu, L.; Wang, Z.; Zhu, J.; Murad, A.L.; Prokop, L.J.; Murad, M.H. Nut consumption and risk of cancer and type 2 diabetes: A systematic review and meta-analysis. Nutr. Rev. 2015, 73, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Hu, F.B.; Giovannucci, E.L.; Wolpin, B.M.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Nut consumption and risk of pancreatic cancer in women. Br. J. Cancer 2013, 109, 2911–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenab, M.; Ferrari, P.; Slimani, N.; Norat, T.; Casagrande, C.; Overad, K.; Olsen, A.; Stripp, C.; Tjonneland, A.; Boutron-Ruault, M.C.; et al. Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1595–1603. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Bullo, M.; Juanola-Falgarona, M.; Hernandez-Alonso, P.; Salas-Salvado, J. Nutrition attributes and health effects of pistachio nuts. Br. J. Nutr. 2015, 113 (Suppl. 2), S79–S93. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Day, N.E.; Luben, R.; Ferrari, P.; Slimani, N.; Norat, T.; Clavel-Chapelon, F.; Kesse, E.; Nieters, A.; Boeing, H.; et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 2003, 361, 1496–1501. [Google Scholar] [CrossRef]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC). PLoS ONE 2012, 7, e39361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [PubMed]
- Goncalves, P.; Martel, F. Butyrate and colorectal cancer: The role of butyrate transport. Curr. Drug Metab. 2013, 14, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Liu, Y.G.; Zou, M.C.; Zou, F. Sodium butyrate induces apoptosis of human colon cancer cells by modulating ERK and sphingosine kinase 2. Biomed. Environ. Sci. 2014, 27, 197–203. [Google Scholar] [PubMed]
- Barnard, J.A.; Warwick, G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 1993, 4, 495–501. [Google Scholar] [PubMed]
- Stuetz, W.; Schlormann, W.; Glei, M. B-vitamins, carotenoids and alpha-/gamma-tocopherol in raw and roasted nuts. Food Chem. 2017, 221, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Shahidi, F. Tree Nuts-Composition, Phytochemicals and Health Effects; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Bullo, M.; Migo-Correig, P.; Marquez-Sandoval, F.; Babio, N.; Martinez-Gonzalez, M.A.; Estruch, R.; Basora, J.; Sola, R.; Salas-Salvado, J. Mediterranean diet and high dietary acid load associated with mixed nuts: Effect on bone metabolism in elderly subjects. J. Am. Geriatr. Soc. 2009, 57, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Max Rubner Institut. Nationale Verzehrsstudie II Ergebnissbericht Teil 2; Max Rubner Institut: Karlsruhe, Germany, 2008. [Google Scholar]
- Alamprese, C.; Ratti, S.; Rossi, M. Effects of roasting conditions on hazelnut characteristics in a two-step process. J. Food Eng. 2009, 95, 272–279. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bencomo, J.J.; Kelebek, H.; Sonmezdag, A.S.; Rodriguez-Alcala, L.M.; Fontecha, J.; Selli, S. Characterization of the Aroma-Active, Phenolic, and Lipid Profiles of the Pistachio (Pistacia vera L.) nut as affected by the single and double roasting process. J. Agric. Food Chem. 2015, 63, 7830–7839. [Google Scholar] [CrossRef] [PubMed]
- Agila, A.; Barringer, S. Effect of roasting conditions on color and volatile profile including HMF level in sweet almonds (prunus dulcis). J. Food Sci. 2012, 77, C461–C468. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Birringer, M.; Böhm, V.; Lober, K.; Jahreis, G.; Lorkowski, S.; Müller, A.K.; Schöne, F.; Glei, M. Influence of roasting conditions on health-related compounds in different nuts. Food Chem. 2015, 180, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Lamberty, J.; Lorkowski, S.; Ludwig, D.; Mothes, H.; Saupe, C.; Glei, M. Chemopreventive potential of in vitro fermented nuts in LT97 colon adenoma and primary epithelial colon cells. Mol. Carcinog. 2017, 56, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Jurek, D.; Wrba, F.; Kaserer, K.; Wurzer, G.; Karner-Hanusch, J.; Marian, B. Cells obtained from colorectal microadenomas mirror early premalignant growth patterns in vitro. Eur. J. Cancer 2002, 38, 1937–1945. [Google Scholar] [CrossRef]
- Schlörmann, W.; Lamberty, J.; Ludwig, D.; Lorkowski, S.; Glei, M. In vitro–fermented raw and roasted walnuts induce expression of CAT and GSTT2 genes, growth inhibition, and apoptosis in LT97 colon adenoma cells. Nutr. Res. 2017, 47, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Glei, M.; Fischer, S.; Lamberty, J.; Ludwig, D.; Lorkowski, S.; Schlörmann, W. Chemopreventive potential of in vitro fermented raw and roasted hazelnuts in LT97 colon adenoma cells. Anticancer Res. 2018, in press. [Google Scholar]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113 (Suppl. 2), S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; Josse, A.R.; Esfahani, A.; Jenkins, D.J. Nuts, metabolic syndrome and diabetes. Br. J. Nutr. 2010, 104, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Kendall, C.W.; Blanco, M.S.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of tree nuts on glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled dietary trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef] [PubMed]
- DGE; ÖGE; SGE; D-A-CH. Referenzwerte für Die Nährstoffzufuhr; DGE: Bonn, Germany, 2015. [Google Scholar]
- World Health Organization (WHO). Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO Study Group; WHO Technical Report Series No. 797; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Amaral, J.S.; Casal, S.; Seabra, R.M.; Oliveira, B.P. Effects of roasting on hazelnut lipids. J. Agric. Food Chem. 2006, 54, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Birringer, M.; Lochner, A.; Lorkowski, S.; Richter, I.; Rohrer, C.; Glei, M. In vitro fermentation of nuts results in the formation of butyrate and c9,t11 conjugated linoleic acid as chemopreventive metabolites. Eur. J. Nutr. 2016, 55, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Borowicki, A.; Stein, K.; Scharlau, D.; Scheu, K.; Brenner-Weiss, G.; Obst, U.; Hollmann, J.; Lindhauer, M.; Wachter, N.; Glei, M. Fermented wheat aleurone inhibits growth and induces apoptosis in human HT29 colon adenocarcinoma cells. Br. J. Nutr. 2010, 103, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [PubMed]
- Pool-Zobel, B.L.; Sauer, J. Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J. Nutr. 2007, 137, 2580S–2584S. [Google Scholar] [PubMed]
- Schlörmann, W.; Hiller, B.; Jahns, F.; Zoger, R.; Hennemeier, I.; Wilhelm, A.; Lindhauer, M.G.; Glei, M. Chemopreventive effects of in vitro digested and fermented bread in human colon cells. Eur. J. Nutr. 2012, 51, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Naumann, S.; Renner, C.; Glei, M. Influence of miRNA-106b and miRNA-135a on butyrate-regulated expression of p21 and Cyclin D2 in human colon adenoma cells. Genes Nutr. 2015, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, J.W.; Lee, J.Y.; Kwon, T.K. Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells. Carcinogenesis 2004, 25, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, H.S.; Xia, H. Sodium butyrate induces human colon carcinoma HT-29 cell apoptosis through a mitochondrial pathway. J. Int. Med. Res. 2009, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.L.; Selvaraju, V.; Sauer, J.; Kautenburger, T.; Kiefer, J.; Richter, K.K.; Soom, M.; Wolfl, S. Butyrate may enhance toxicological defence in primary, adenoma and tumor human colon cells by favourably modulating expression of glutathione S-transferases genes, an approach in nutrigenomics. Carcinogenesis 2005, 26, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, X.; Zhang, W.; Zhang, G.; Nguyen, A.K.; Liu, X.; Jimenez, F.; Cox, C.S., Jr.; Townsend, C.M., Jr.; Ko, T.C. Dietary fiber enhances TGF-beta signaling and growth inhibition in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G156–G164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Huang, Y.X.; Sun, Y.; Zheng, L.H.; Li, Y.X. Butyrate induces cell apoptosis through activation of JNK MAP kinase pathway in human colon cancer RKO cells. Chem. Biol. Interact. 2010, 185, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, D.; Lee, A.; Wong, T.; Marian, B.; Chiaro, C.; Rainey, C.; Bordonaro, M. Modulation of Wnt activity and cell physiology by Butyrate in LT97 Microadenoma Cells. J. Cancer 2014, 5, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Periago, P.; Espin, J.C.; Tomas-Barberan, F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid. Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Nunez-Sanchez, M.A.; Tome-Carneiro, J.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancer and key-associated molecular hallmarks: MicroRNA cell specific induction of CDKN1A (p21) as a common mechanism involved. Mol. Nutr. Food Res. 2016, 60, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Sanchez, M.A.; Karmokar, A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Brown, K.; Espin, J.C. In vivo relevant mixed urolithins and ellagic acid inhibit phenotypic and molecular colon cancer stem cell features: A new potentiality for ellagitannin metabolites against cancer. Food Chem. Toxicol. 2016, 92, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, J.; Xu, T.J.; Qin, X.Y.; Zhou, X.D. Sodium selenite induces apoptosis in colon cancer cells via Bax-dependent mitochondrial pathway. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2166–2171. [Google Scholar] [PubMed]
- Rudolf, E.; Kralova, V.; Cervinka, M. Selenium and colon cancer—From chemoprevention to new treatment modality. Anticancer Agents Med. Chem. 2008, 8, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Glei, M.; Schneider, T.; Schlörmann, W. Comet assay: An essential tool in toxicological research. Arch. Toxicol. 2016, 90, 2315–2336. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Chen, C.Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.N.; Beyer-Sehlmeyer, G.; Liegibel, U.M.; Kautenburger, T.; Becker, T.W.; Pool-Zobel, B.L. Butyrate induces glutathione S-transferase in human colon cells and protects from genetic damage by 4-hydroxy-2-nonenal. Nutr. Cancer 2001, 41, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Borowicki, A.; Scharlau, D.; Glei, M. Fermented wheat aleurone induces enzymes involved in detoxification of carcinogens and in antioxidative defence in human colon cells. Br. J. Nutr. 2010, 104, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Shu, L.; Khor, T.O.; Lee, J.H.; Fuentes, F.; Kong, A.N. A perspective on dietary phytochemicals and cancer chemoprevention: Oxidative stress, nrf2, and epigenomics. Top. Curr. Chem. 2013, 329, 133–162. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glei, M.; Ludwig, D.; Lamberty, J.; Fischer, S.; Lorkowski, S.; Schlörmann, W. Chemopreventive Potential of Raw and Roasted Pistachios Regarding Colon Carcinogenesis. Nutrients 2017, 9, 1368. https://doi.org/10.3390/nu9121368
Glei M, Ludwig D, Lamberty J, Fischer S, Lorkowski S, Schlörmann W. Chemopreventive Potential of Raw and Roasted Pistachios Regarding Colon Carcinogenesis. Nutrients. 2017; 9(12):1368. https://doi.org/10.3390/nu9121368
Chicago/Turabian StyleGlei, Michael, Diana Ludwig, Julia Lamberty, Sonja Fischer, Stefan Lorkowski, and Wiebke Schlörmann. 2017. "Chemopreventive Potential of Raw and Roasted Pistachios Regarding Colon Carcinogenesis" Nutrients 9, no. 12: 1368. https://doi.org/10.3390/nu9121368




