A Correlation Study of DHA Intake Estimated by a FFQ and Concentrations in Plasma and Erythrocytes in Mid- and Late Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Dietary Assessment
2.3. Blood Sample Collection and Analysis
2.4. Statistical Analysis
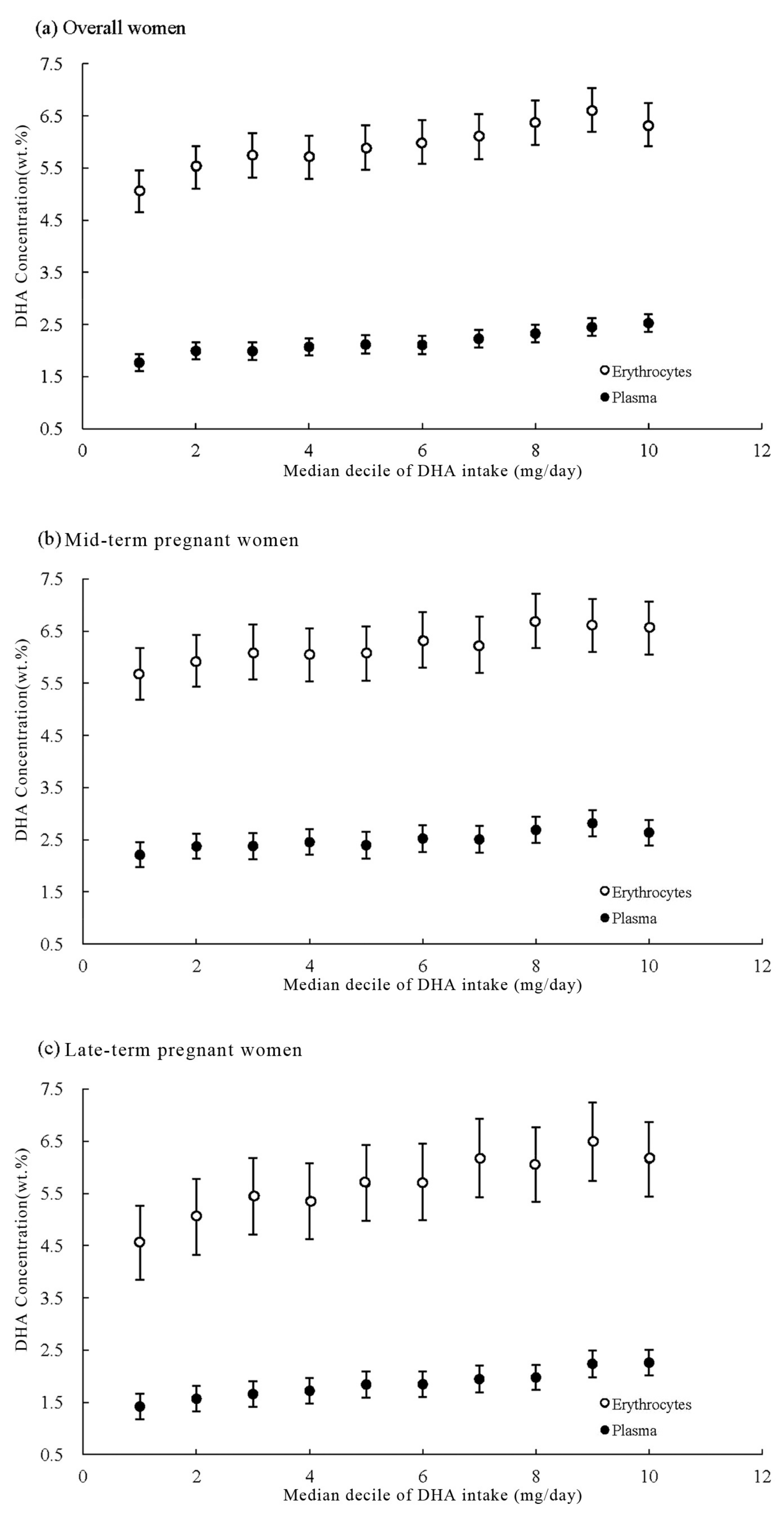
3. Results
3.1. Maternal Characteristics
3.2. DHA Dietary Intake and Concentrations
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am. J. Clin. Nutr. 2000, 71, 307S–311S. [Google Scholar] [PubMed]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Goyens, P.L.; Mensink, R.P. Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur. J. Clin. Nutr. 2006, 60, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13C] linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid. Res. 2005, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO) of the United Nations. Fat and Fatty Acids in Human Nutrition: Report of an Expert Consultation; Food and Agriculture Organization: Rome, Italy, 2010. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Cetin, I.; Brenna, J.T. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B. Nutritional epidemiology-past, present, future. Int. J. Epidemiol. 2003, 32, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [PubMed]
- Andersen, L.F.; Solvoll, K.; Johansson, L.R.; Salminen, I.; Aro, A.; Drevon, C.A. Evaluation of a food frequency questionnaire with weighed records, fatty acids, and alpha-tocopherol in adipose tissue and serum. Am. J. Epidemiol. 1999, 150, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Ovesen, L.; Bülow, I.; Jørgensen, T.; Knudsen, N.; Laurberg, P.; Perrild, H. Evaluation of a semi-quantitative food frequency questionnaire to estimate iodine intake. Eur. J. Clin. Nutr. 2001, 55, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Haugen, M.; Thomassen, Y.; Ellingsen, D.G.; Ydersbond, T.A.; Hagve, T.A.; Alexander, J.; Meltzer, H.M. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010, 13, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Elias, S.L. Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women. Am. J. Clin. Nutr. 2003, 77, 473–478. [Google Scholar] [PubMed]
- Kobayashi, M.; Jwa, S.C.; Ogawa, K.; Morisaki, N.; Fujiwara, T. Validity of a food frequency questionnaire to estimate long-chain polyunsaturated fatty acid intake among Japanese women in early and late pregnancy. J. Epidemiol. 2017, 27, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lepsch, J.; Vaz, J.S.; Moreira, J.D.; Pinto, T.J.; Soares, M.M.; Kac, G. Food frequency questionnaire as an indicator of the serum composition of essential n-3 and n-6 polyunsaturated fatty acids in early pregnancy, according to body mass index. J. Hum. Nutr. Diet. 2015, 28, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.; McClure, G.; Hegarty, B.D.; Smith, I.G. The validity of a food frequency questionnaire as a measure of PUFA status in pregnancy. BMC Pregnancy Childbirth 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.S.; Schnaas, L.; Meydani, M.; Perroni, E.; Martínez, S.; Romieu, I. Erythrocyte cell membrane phospholipid levels compared against reported dietary intakes of polyunsaturated fatty acids in pregnant Mexican women. Public Health Nutr. 2002, 5, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Meng, L.; Wang, C.; Zhao, W.; Chen, J.; Ghebremeskel, K.; Crawford, M.A. Maternal and neonatal plasma n-3 and n-6 fatty acids of pregnant women and neonates in three regions in China with contrasting dietary patterns. Asia Pac. J. Clin. Nutr. 2009, 18, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cade, J.; Jackson, M.; Mbanya, J.C.; Chungong, S.; Forrester, T.; Bennett, F.; Wilks, R.; Balkau, B.; Cruickshank, J.K. Development of food frequency questionnaires in three population samples of African origin from Cameroon, Jamaica and Caribbean migrants to the UK. Eur. J. Clin. Nutr. 1996, 50, 479–486. [Google Scholar] [PubMed]
- Yang, X.G.; Zhai, F.Y. China Nutrition and Health Survey in 2002, Book II-Food and Nutrients Intake; People’s Medical Publishing House: Beijing, China, 2006; pp. 1–278. [Google Scholar]
- Al, M.D.; van Houwelingen, A.C.; Kester, A.D.; Hasaart, T.H.; de Jong, A.E.; Hornstra, G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br. J. Nutr. 1995, 74, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.J.; van Houwelingen, A.C.; Badart-Smook, A.; Hornstra, G. Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. Am. J. Clin. Nutr. 2001, 73, 302–307. [Google Scholar] [PubMed]
- Liu, M.J.; Li, H.T.; Yu, L.X.; Xu, G.S.; Ge, H.; Wang, L.L.; Zhang, Y.L.; Zhou, Y.B.; Li, Y.; Bai, M.X.; et al. A Correlation Study of DHA Dietary Intake and Plasma, Erythrocyte and Breast Milk DHA Concentrations in Lactating Women from Coastland, Lakeland, and Inland Areas of China. Nutrients 2016, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Wang, G.Y. China Food Compotion Table, 2nd ed.; Peking University Medical Press: Beijing, China, 2009; pp. 271–297. [Google Scholar]
- Li, Y.; Li, H.T.; Trasande, L.; Ge, H.; Yu, L.X.; Xu, G.S.; Bai, M.X.; Liu, J.M. DHA in Pregnant and Lactating Women from Coastland, Lakeland, and Inland Areas of China: Results of a DHA Evaluation in Women (DEW) Study. Nutrients 2015, 7, 8723–8732. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, K.M.; Lipsitz, S.R.; Linder, J.A.; Rimm, E.; Hoel, D.G. Using median regression to obtain adjusted estimates of central tendency for skewed laboratory and epidemiologic data. Clin. Chem. 2009, 55, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Ramos, E.; Severo, M.; Casal, S.; Dos Santos Silva, I.; Lopes, C.; Barros, H. Measurement of dietary intake of fatty acids in pregnant women: Comparison of self-reported intakes with adipose tissue levels. Ann. Epidemiol. 2010, 20, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Sacks, F.M.; Colditz, G.A.; Rimm, E.B.; Sampson, L.A.; Willett, W.C.; Hunter, D.J. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am. J. Clin. Nutr. 1998, 67, 25–30. [Google Scholar] [PubMed]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Blanco, J.E.; Pagán, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 1908S–1913S. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.M. Assessment of individual fatty acid intake. Proc. Nutr. Soc. 2000, 59, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Gao, Y.; Li, L.; Man, Q.; Song, P.; Meng, L.; Du, Z.Y.; Miles, E.A.; Lie, Ø.; et al. Different intakes of n-3 fatty acids among pregnant women in 3 regions of China with contrasting dietary patterns are reflected in maternal but not in umbilical erythrocyte phosphatidylcholine fatty acid composition. Nutr. Res. 2013, 33, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Heim, T.; Swyer, P.R.; Chance, G.W. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum. Dev. 1981, 5, 355–366. [Google Scholar] [CrossRef]
- Innis, S.M. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003, 143, S1–S8. [Google Scholar] [CrossRef]
| Characteristics | Overall (N = 804) | Mid-Pregnancy (N = 407) | Late Pregnancy (N = 397) | p Value 1 | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (years) | 0.065 | ||||||
| ≤25 | 140 | 17.4 | 75 | 18.4 | 65 | 16.4 | |
| >25 to 30 | 491 | 61.1 | 258 | 63.4 | 233 | 58.7 | |
| >30 | 173 | 21.5 | 74 | 18.2 | 99 | 24.9 | |
| Pre-pregnancy BMI (kg/m2) | 0.235 | ||||||
| <18.5 | 147 | 18.3 | 74 | 18.2 | 73 | 18.4 | |
| 18.5 to <25 | 591 | 73.5 | 293 | 72.0 | 298 | 75.1 | |
| ≥25 | 66 | 8.2 | 40 | 9.8 | 26 | 6.6 | |
| Area of residence | 0.904 | ||||||
| Coastland | 263 | 32.7 | 136 | 33.4 | 127 | 32.0 | |
| Lakeland | 267 | 33.2 | 133 | 32.7 | 134 | 33.8 | |
| Inland | 274 | 34.1 | 138 | 33.9 | 136 | 34.3 | |
| Parity | 0.317 | ||||||
| Nulliparous | 665 | 82.7 | 342 | 84.0 | 323 | 81.4 | |
| Multiparous | 139 | 17.3 | 65 | 16.0 | 74 | 18.6 | |
| Ethnicity | 0.012 | ||||||
| Han | 764 | 95.0 | 379 | 93.1 | 385 | 97.0 | |
| Others | 40 | 5.0 | 28 | 6.9 | 12 | 3.0 | |
| Education | 0.994 | ||||||
| College or above | 523 | 65.1 | 264 | 64.8 | 259 | 65.2 | |
| High school | 175 | 21.8 | 89 | 21.9 | 86 | 21.7 | |
| Middle school or less | 106 | 13.2 | 54 | 13.3 | 52 | 13.1 | |
| Annual family income per capita (Yuan) | 0.050 | ||||||
| <30,000 | 296 | 36.8 | 151 | 37.1 | 145 | 36.5 | |
| 30,000 to <50,000 | 231 | 28.7 | 125 | 30.7 | 106 | 26.7 | |
| 50,000 to <100,000 | 191 | 23.8 | 85 | 20.9 | 106 | 26.7 | |
| ≥100,000 | 30 | 3.7 | 11 | 2.7 | 19 | 4.8 | |
| Missing | 56 | 7.0 | 35 | 8.6 | 21 | 5.3 | |
| Overall | Pregnancy Stages | Regions | ||||||
|---|---|---|---|---|---|---|---|---|
| Mid-Pregnancy | Late Pregnancy | p-Value | Coastland | Lakeland | Inland | p-Value 1 | ||
| Dietary intake (mg/day) | ||||||||
| Median | 18.9 | 18.1 | 20.0 | 0.429 | 28.6 | 22.3 | 9.1 | <0.001 |
| IQR | 7.8 to 45.1 | 7.5 to 41.8 | 7.9 to 47.7 | 15.0 to 64.4 | 10.4 to 44.8 | 2.6 to 23.0 | ||
| Adjusted median 2 | 20.2 | 19.4 | 21.7 | 29.1 | 20.3 | 9.3 | ||
| Adjusted IQR 2 | 8.2 to 45.1 | 8.3 to 42.0 | 9.5 to 48.4 | 14.6 to 57.9 | 10.7 to 42.6 | 2.7 to 21.8 | ||
| Minimum, maximum | 0.0 to 850.9 | 0.0 to 348.8 | 0.0 to 850.9 | 0.2 to 850.9 | 0.0 to 348.8 | 0.0 to 425.6 | ||
| Mean | 38.3 | 34.0 | 42.6 | 50.7 | 39.1 | 25.6 | ||
| SD | 59.6 | 45.9 | 70.1 | 70.7 | 51.5 | 52.4 | ||
| Plasma (wt. %) | ||||||||
| Median | 2.3 | 2.6 | 2.0 | <0.001 | 2.8 | 2.2 | 1.9 | <0.001 |
| IQR | 1.9 to 2.7 | 2.2 to 3.0 | 1.6 to 2.3 | 2.4 to 3.3 | 1.9 to 2.5 | 1.6 to 2.3 | ||
| Adjusted median 2 | 2.2 | 2.6 | 2.0 | 2.8 | 2.2 | 2.0 | ||
| Adjusted IQR 2 | 1.9 to 2.6 | 2.2 to 3.1 | 1.6 to 2.4 | 2.4 to 3.3 | 1.9 to 2.5 | 1.6 to 2.3 | ||
| Minimum, maximum | 0.8 to 5.4 | 1.1 to 5.4 | 0.8 to 4.3 | 1.4 to 5.4 | 0.9 to 4.0 | 0.8 to 3.6 | ||
| Mean | 2.3 | 2.6 | 2.0 | 2.9 | 2.2 | 2.0 | ||
| SD | 0.7 | 0.7 | 0.6 | 0.7 | 0.5 | 0.5 | ||
| Erythrocyte (wt. %) | ||||||||
| Median | 6.3 | 6.5 | 6.1 | <0.001 | 7.5 | 6.3 | 5.5 | <0.001 |
| IQR | 5.5 to 7.3 | 5.7 to 7.5 | 5.3 to 7.1 | 6.7 to 8.5 | 5.8 to 6.9 | 5.0 to 6.2 | ||
| Adjusted median 2 | 6.5 | 6.6 | 6.3 | 7.6 | 6.2 | 5.5 | ||
| Adjusted IQR 2 | 5.4 to 7.4 | 5.9 to 7.5 | 5.4 to 7.3 | 6.9 to 8.3 | 5.7 to 6.8 | 5.1 to 6.2 | ||
| Minimum, maximum | 0.7 to 11.2 | 0.8 to 10.2 | 0.7 to 11.2 | 0.7 to 11.2 | 2.1 to 9.4 | 1.0 to 8.4 | ||
| Mean | 6.4 | 6.6 | 6.1 | 7.4 | 6.3 | 5.5 | ||
| SD | 1.5 | 1.4 | 1.7 | 1.7 | 1.0 | 1.2 | ||
| Plasma | Erythrocytes | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude | p | Partial 1 | p | Crude | p | Partial 1 | p | |
| Overall | 0.32 | <0.001 | 0.35 | <0.001 | 0.34 | <0.001 | 0.33 | <0.001 |
| Pregnancy stages | ||||||||
| Mid-pregnancy | 0.27 | <0.001 | 0.25 | <0.001 | 0.30 | <0.001 | 0.26 | <0.001 |
| Late pregnancy | 0.45 | <0.001 | 0.44 | <0.001 | 0.38 | <0.001 | 0.39 | <0.001 |
| Regions | ||||||||
| Coastland | 0.19 | 0.007 | 0.32 | <0.001 | 0.04 | 0.534 | 0.06 | 0.335 |
| Lakeland | 0.16 | 0.010 | 0.16 | 0.009 | 0.24 | <0.001 | 0.23 | <0.001 |
| Inland | 0.20 | <0.001 | 0.17 | 0.005 | 0.29 | <0.001 | 0.27 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-B.; Li, H.-T.; Trasande, L.; Wang, L.-L.; Zhang, Y.-L.; Si, K.-Y.; Bai, M.-X.; Liu, J.-M. A Correlation Study of DHA Intake Estimated by a FFQ and Concentrations in Plasma and Erythrocytes in Mid- and Late Pregnancy. Nutrients 2017, 9, 1256. https://doi.org/10.3390/nu9111256
Zhou Y-B, Li H-T, Trasande L, Wang L-L, Zhang Y-L, Si K-Y, Bai M-X, Liu J-M. A Correlation Study of DHA Intake Estimated by a FFQ and Concentrations in Plasma and Erythrocytes in Mid- and Late Pregnancy. Nutrients. 2017; 9(11):1256. https://doi.org/10.3390/nu9111256
Chicago/Turabian StyleZhou, Yu-Bo, Hong-Tian Li, Leonardo Trasande, Lin-Lin Wang, Ya-Li Zhang, Ke-Yi Si, Man-Xi Bai, and Jian-Meng Liu. 2017. "A Correlation Study of DHA Intake Estimated by a FFQ and Concentrations in Plasma and Erythrocytes in Mid- and Late Pregnancy" Nutrients 9, no. 11: 1256. https://doi.org/10.3390/nu9111256




