High Neonatal Blood Iron Content Is Associated with the Risk of Childhood Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
2.2. Sample Collection
2.3. Analysis of Iron and HLA-DQB1 on NDBSs
2.4. Descriptive Data
2.5. Statistical Analyses
3. Results
3.1. Basic Characteristics
3.2. Iron and T1D Risk
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Svensson, J.; Lyngaae-Jørgensen, A.; Carstensen, B.; Simonsen, L.B.; Mortensen, H.B. Danish Childhood Diabetes Registry Long-term trends in the incidence of type 1 diabetes in Denmark: The seasonal variation changes over time. Pediatr. Diabetes 2009, 10, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Dahlquist, G.G.; Gyürüs, E.; Green, A.; Soltész, G.; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Pietropaolo, M.; Towns, R.; Eisenbarth, G.S. Humoral Autoimmunity in Type 1 Diabetes: Prediction, Significance, and Detection of Distinct Disease Subtypes. Cold Spring Harb. Perspect. Med. 2012, 2, a012831. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Bain, S.C.; Barnett, A.H.; Bingley, P.J.; Christie, M.R.; Gill, G.V.; Gale, E.A. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004, 364, 1699–1700. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal programming and adult health. Public Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.W.; Maruoka, S.; Boon, K.; Garantziotis, S.; Li, Z.; Tomfohr, J.; Bailey, N.; Potts, E.N.; Whitehead, G.; Brass, D.M.; et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J. Clin. Investig. 2008, 118, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Saha, K.; Mukhopadhyay, D.; Roy, S.; Raychaudhuri, G.; Chatterjee, M.; Mitra, P.K. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: A case control study. J. Nat. Sci. Biol. Med. 2014, 5, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L. The role of iron in T cell development and autoimmunity. Autoimmun. Rev. 2003, 2, 73–78. [Google Scholar] [CrossRef]
- Ellervik, C.; Mandrup-Poulsen, T.; Nordestgaard, B.G.; Larsen, L.E.; Appleyard, M.; Frandsen, M.; Petersen, P.; Schlichting, P.; Saermark, T.; Tybjaerg-Hansen, A.; et al. Prevalence of hereditary haemochromatosis in late-onset type 1 diabetes mellitus: A retrospective study. Lancet 2001, 358, 1405–1409. [Google Scholar] [CrossRef]
- Saudek, C.D.; Hemm, R.M.; Peterson, C.M. Abnormal glucose tolerance in beta-thalassemia major. Metabolism 1977, 26, 43–52. [Google Scholar] [CrossRef]
- Ellervik, C.; Mandrup-Poulsen, T.; Andersen, H.U.; Tybjærg-Hansen, A.; Frandsen, M.; Birgens, H.; Nordestgaard, B.G. Elevated Transferrin Saturation and Risk of Diabetes. Diabetes Care 2011, 34, 2256–2258. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and Diabetes Risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.I.; Okam, M.M. Anemia in pregnancy. Hematol. Oncol. Clin. N. Am. 2011, 25, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, V.K.; Hansen, H.S.; Ovesen, L.; Mikkelsen, T.B.; Olsen, S.F. Iron supplement use among Danish pregnant women. Public Health Nutr. 2007, 10, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lou, J.; Rao, R.; Georgieff, M.K.; Kaciroti, N.; Felt, B.T.; Zhao, Z.-Y.; Lozoff, B. Maternal Serum Ferritin Concentration Is Positively Associated with Newborn Iron Stores in Women with Low Ferritin Status in Late Pregnancy. J. Nutr. 2012, 142, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, C.; Mishra, S.; Ajmera, P.; Sharma, P. Serum iron, copper and zinc status in maternal and cord blood. Indian J. Clin. Biochem. 2004, 19, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard-Pedersen, B.; Hougaard, D.M. Storage policies and use of the Danish Newborn Screening Biobank. J. Inherit. Metab. Dis. 2007, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Eising, S.; Svensson, J.; Skogstrand, K.; Nilsson, A.; Lynch, K.; Andersen, P.S.; Lernmark, Å.; Hougaard, D.M.; Pociot, F.; Nørgaard-Pedersen, B.; et al. Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: Design and feasibility of an unselected case–control study. Paediatr. Perinat. Epidemiol. 2007, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Capiau, S.; Stove, V.V.; Lambert, W.E.; Stove, C.P. Prediction of the hematocrit of dried blood spots via potassium measurement on a routine clinical chemistry analyzer. Anal. Chem. 2013, 85, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.S.; Zoriy, M.; Matusch, A.; Wu, B.; Salber, D.; Palm, C.; Becker, J.S. Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom. Rev. 2010, 29, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; Sanz-Medel, A. Laser ablation ICP-MS for quantitative biomedical applications. Anal. Bioanal. Chem. 2012, 403, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Thomson, G.; Valdes, A.M.; Noble, J.A.; Kockum, I.; Grote, M.N.; Najman, J.; Erlich, H.A.; Cucca, F.; Pugliese, A.; Steenkiste, A.; et al. Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: A meta-analysis. Tissue Antigens 2007, 70, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.P.; Eason, N.B.; Kabagambe, E.K.; Haritha, J.; Meleth, S.; McCormick, K.L. Dietary iron intake in the first 4 months of infancy and the development of type 1 diabetes: A pilot study. Diabetol. Metab. Syndr. 2010, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- Patelarou, E.; Girvalaki, C.; Brokalaki, H.; Patelarou, A.; Androulaki, Z.; Vardavas, C. Current evidence on the associations of breastfeeding, infant formula, and cow’s milk introduction with type 1 diabetes mellitus: A systematic review. Nutr. Rev. 2012, 70, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, A.; Van Campenhout, C.; Lagrou, A.R.; Abrams, P.; Moorkens, G.; Van Gaal, L.; Manuel-y-Keenoy, B. Impact of diabetes mellitus on the relationships between iron-, inflammatory- and oxidative stress status. Diabetes Metab. Res. Rev. 2006, 22, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Bocca, B.; Peruzzu, A.; Tolu, F.; Asara, Y.; Farace, C.; Oggiano, R.; Madeddu, R. Blood metals concentration in type 1 and type 2 diabetics. Biol. Trace Elem. Res. 2013, 156, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Fleming, R.E. Bone Morphogenetic Proteins as Regulators of Iron Metabolism. Annu. Rev. Nutr. 2014, 34, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Neghina, A.M.; Anghel, A. Hemochromatosis Genotypes and Risk of Iron Overload—A Meta-Analysis. Ann. Epidemiol. 2011, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.F.S.; Silva, M.; Dornas, W.C.; Costa, D.C.; Silva, M.E.; Dos Santos, R.C.; de Lima, W.G.; Pedrosa, M.L. Iron toxicity mediated by oxidative stress enhances tissue damage in an animal model of diabetes. Biometals 2014, 27, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Lunova, M.; Schwarz, P.; Nuraldeen, R.; Levada, K.; Kuscuoglu, D.; Stützle, M.; Vujić Spasić, M.; Haybaeck, J.; Ruchala, P.; Jirsa, M.; et al. Hepcidin knockout mice spontaneously develop chronic pancreatitis owing to cytoplasmic iron overload in acinar cells. J. Pathol. 2017, 241, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, S.; Grgurevic, L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.D.; Marrero, I.G.; Gros, P.; Zaghouani, H.; Wicker, L.S.; Sercarz, E.E. Slc11a1 Enhances the Autoimmune Diabetogenic T-Cell Response by Altering Processing and Presentation of Pancreatic Islet Antigens. Diabetes 2009, 58, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B.; Tonnesen, M.F.; Madsen, A.N.; Hagedorn, P.H.; Friberg, J.; Grunnet, L.G.; Heller, R.S.; Nielsen, A.Ø.; Størling, J.; Baeyens, L.; et al. Divalent Metal Transporter 1 Regulates Iron-Mediated ROS and Pancreatic β Cell Fate in Response to Cytokines. Cell Metab. 2012, 16, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Coffey, R.; Knutson, M.D. The plasma membrane metal-ion transporter ZIP14 contributes to non-transferrin-bound iron uptake by human β cells. Am. J. Physiol. Cell Physiol. 2017, 312, C169–C175. [Google Scholar] [CrossRef] [PubMed]
- Trigwell, S.M.; Radford, P.M.; Page, S.R.; Loweth, A.C.; James, R.F.L.; Morgan, N.G.; Todd, I. Islet glutamic acid decarboxylase modified by reactive oxygen species is recognized by antibodies from patients with type 1 diabetes mellitus. Clin. Exp. Immunol. 2001, 126, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Haschka, D.; Demetz, E.; Weiss, G. Iron at the interface of immunity and infection. Front. Pharmacol. 2014, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O. The gut as a regulator of early inflammation in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.; Domellöf, M.; Andersson, D.; Hellström-Westas, L. Effect of Delayed vs. Early Umbilical Cord Clamping on Iron Status and Neurodevelopment at Age 12 Months: A Randomized Clinical Trial. JAMA Pediatr. 2014, 168, 547. [Google Scholar] [CrossRef] [PubMed]
- Wish, J.B. Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin. J. Am. Soc. Nephrol. 2006, 1 (Suppl. 1), S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Waalen, J.; Felitti, V.J.; Gelbart, T.; Beutler, E. Screening for hemochromatosis by measuring ferritin levels: A more effective approach. Blood 2008, 111, 3373–3376. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-F.; Chang, W.-S.; Hsieh, Y.-K.; Wang, C.-F. Using dried-droplet laser ablation inductively coupled plasma mass spectrometry to quantify multiple elements in whole blood. Anal. Chim. Acta 2011, 699, 6–10. [Google Scholar] [CrossRef] [PubMed]
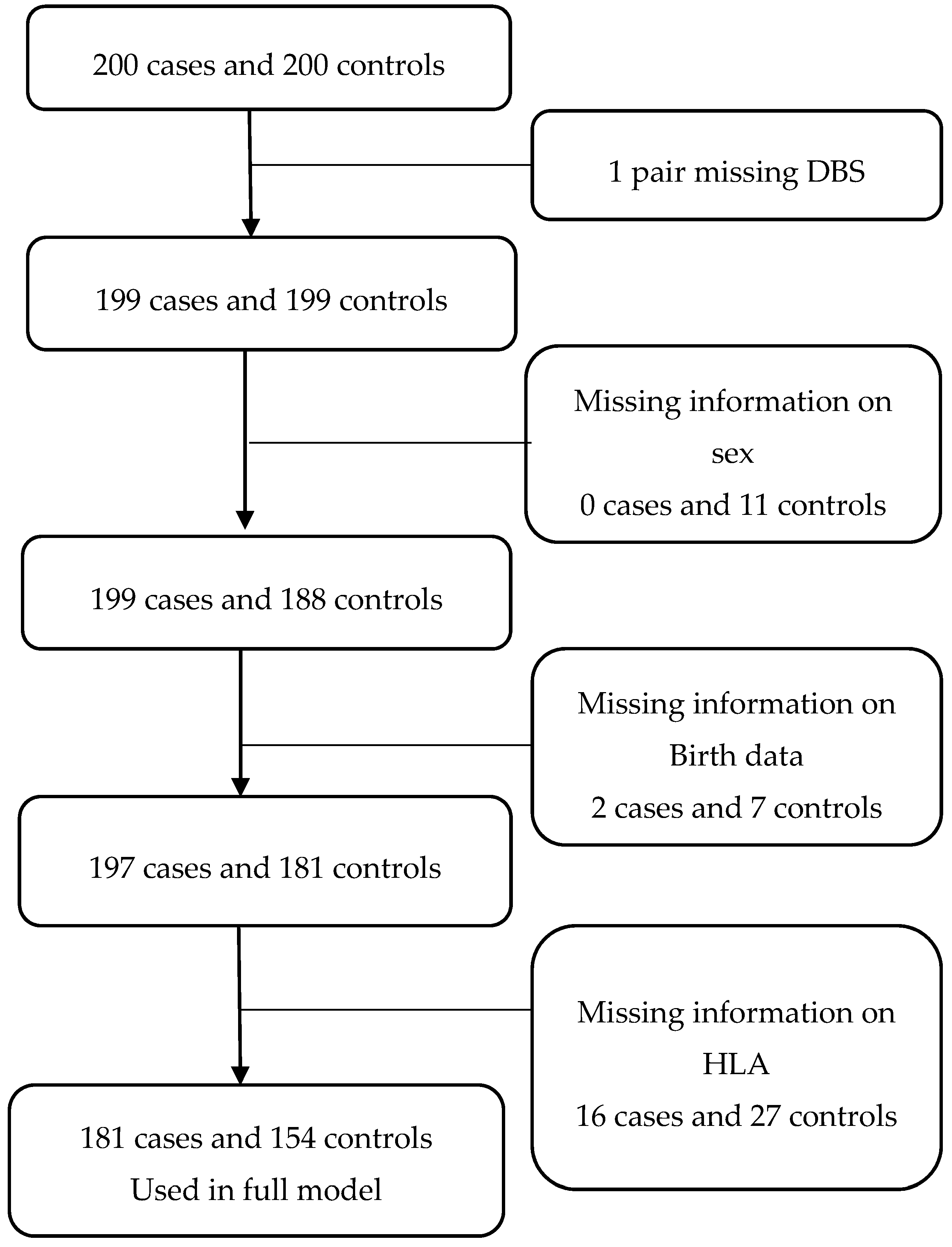
| Variables (Median/Range) | Cases (n = 198) | Controls (n = 188) | p Value | Cases (n = 181) | Controls (n = 154) | p-Value | Missing Data (n/% of Total) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Males (n/% of total) | 96 (48.2) | 92 (46.5) | 0.81 | 87 (48.1) | 78 (50.7) | 0.81 | 11/2.8 |
| Gestational age, weeks (median/range) | 40 (31–43) | 40 (27–43) | 0.08 | 40 (31–43) | 40 (28–43) | 0.22 | 17/4.3 |
| Birth length, cm (median/range) | 52 (42–58) | 52 (35–58) | 0.30 | 52 (42–57) | 52 (35–58) | 0.18 | 21/5.3 |
| Birth weight, kg (median/range) | 3.50 (1.70–5.07) | 3.50 (1.05–4.75) | 0.51 | 3500 (1.70–5.07) | 3.48 (1.05–4.75) | 0.31 | 18/4.5 |
| Maternal age at delivery, years (median/range) | 29 (18–44) | 28 (17–40) | 0.13 | 29 (18–44) | 28 (17–38) | 0.12 | 11/2.8 |
| Birth year | 1993 (1991–1998) | 1993 (1991–1998) | 0.90 | 1993 (1991–1998) | 1993 (1991–1998) | 12/3.0 | |
| HLA-DQB1 alleles | |||||||
| High risk n (%) | 118 (59.3) | 26 (13.9) | 116 (64.1) | 26 (16.9) | |||
| Moderate risk | 37 (18.6) | 39 (20.3) | 37 (20.4) | 37 (24.0) | |||
| Low or protective risk | 28 (14.1) | 104 (51.3) | 28 (15.5) | 91 (59.1) | 45/11.3 | ||
| missing | 16 (8.0) | 29 (14.4) | <0.001 | <0.0001 |
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p-Value | |
| Iron content (doubling) | 2.01 (1.04; 3.87) | 0.032 | 2.55 (1.04; 6.24) | 0.041 |
| Other factors | ||||
| Sex | ||||
| Girls | 1.04 (0.69; 1.55) | 0.89 | 1.23 (0.72; 2.08) | 0.45 |
| Boys | 1 | 1 | ||
| Gestational age (weeks) | 0.94 (0.84; 1.06) | 0.31 | 0.81 (0.68; 0.97) | 0.022 |
| Birth weight (100 g) | 1.02 (0.98; 1.06) | 0.34 | 1.04 (0.98; 1.10) | 0.22 |
| Maternal age (year) | 1.03 (0.98; 1.08) | 0.19 | 1.00 (0.95; 1.06) | 0.90 |
| HLA-DQB1 alleles | ||||
| High risk | 17.2 (9.42; 31.3) | 15.8 (8.38; 29.6) | ||
| Moderate risk | 3.44 (1.85; 6.39) | 3.60 (1.88; 6.88) | ||
| Low risk | 1 | <0.0001 | 0.0001 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyvsgaard, J.N.; Overgaard, A.J.; Thorsen, S.U.; Hansen, T.H.; Pipper, C.B.; Mortensen, H.B.; Pociot, F.; Svensson, J. High Neonatal Blood Iron Content Is Associated with the Risk of Childhood Type 1 Diabetes Mellitus. Nutrients 2017, 9, 1221. https://doi.org/10.3390/nu9111221
Kyvsgaard JN, Overgaard AJ, Thorsen SU, Hansen TH, Pipper CB, Mortensen HB, Pociot F, Svensson J. High Neonatal Blood Iron Content Is Associated with the Risk of Childhood Type 1 Diabetes Mellitus. Nutrients. 2017; 9(11):1221. https://doi.org/10.3390/nu9111221
Chicago/Turabian StyleKyvsgaard, Julie Nyholm, Anne Julie Overgaard, Steffen Ullitz Thorsen, Thomas Hesselhøj Hansen, Christian Bressen Pipper, Henrik Bindesbøl Mortensen, Flemming Pociot, and Jannet Svensson. 2017. "High Neonatal Blood Iron Content Is Associated with the Risk of Childhood Type 1 Diabetes Mellitus" Nutrients 9, no. 11: 1221. https://doi.org/10.3390/nu9111221





