Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Bone Mechanical Property Studies
2.3. Bone Mineralization Studies
2.4. Histomorphometric Studies
2.5. Biochemical Studies
2.6. Statistical Analysis
3. Results
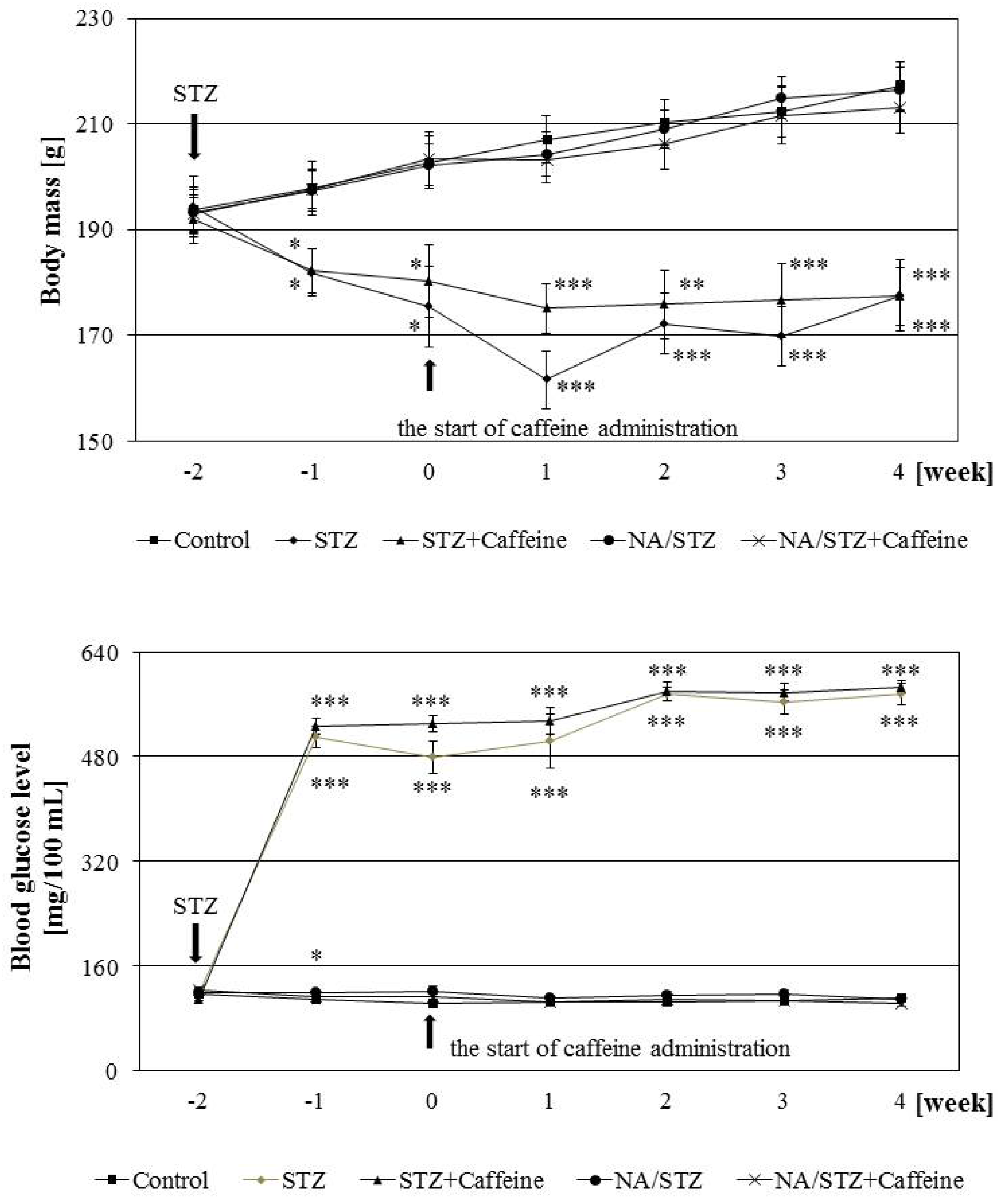
3.1. Effect of Caffeine on the Skeletal System of Rats with Streptozotocin-Induced Diabetes
3.2. Effect of Caffeine on the Skeletal System of Rats Treated with Nicotinamide and Streptozotocin
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2017, 40 (Suppl. S1), S11–S24. [Google Scholar] [CrossRef]
- Yan, W.; Li, X. Impact of diabetes and its treatments on skeletal diseases. Front. Med. 2013, 7, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Vestergaard, P. Management of endocrine disease. Diabetes and osteoporosis: Cause for concern? Eur. J. Endocrinol. 2015, 173, R93–R99. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, E.; Popescu, I.; Bunn, R.C.; Fowlkes, J.L.; Thrailkill, K.M. Effects of type 1 diabetes on osteoblasts, osteocytes, and osteoclasts. Curr. Osteoporos. Rep. 2016, 14, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; D’Onofrio, L.; Buzzetti, R.; Manfrini, S.; Napoli, N. Pathophysiology of bone fragility in patients with diabetes. Calcif. Tissue Int. 2017, 100, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. New insights for oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.S.; Fraser, L.A. Type 1 diabetes and osteoporosis: From molecular pathways to bone phenotype. J. Osteoporos. 2015, 2015, 174186. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, V.V.; Mitchell, D.M.; Rosen, C.J.; Bouxsein, M.L. Type 2 diabetes and the skeleton: New insights into sweet bones. Lancet Diabetes Endocrinol. 2016, 4, 159–173. [Google Scholar] [CrossRef]
- Oei, L.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H. Diabetes, diabetic complications, and fracture risk. Curr. Osteoporos. Rep. 2015, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Masiello, P. Animal models of type 2 diabetes with reduced pancreatic β-cell mass. Int. J. Biochem. Cell Biol. 2006, 38, 873–893. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.S.; Graham, T.E. Methylxanthines and human health: Epidemiological and experimental evidence. Handb. Exp. Pharmacol. 2011, 200, 509–548. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, D.; Jiang, W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Byberg, L.; Glynn, A.; Lemming, E.W.; Wolk, A.; Michaëlsson, K. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am. J. Epidemiol. 2013, 178, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Wolk, A.; Glynn, A.; Michaëlsson, K.; Byberg, L. Coffee consumption and risk of fracture in the Cohort of Swedish Men (COSM). PLoS ONE 2014, 9, e97770. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, X.Z.; Zhang, K.; Tang, Z. Associations between frequency of coffee consumption and osteoporosis in Chinese postmenopausal women. Int. J. Clin. Exp. Med. 2015, 8, 15958–15966. [Google Scholar] [PubMed]
- Hirata, H.; Kitamura, K.; Saito, T.; Kobayashi, R.; Iwasaki, M.; Yoshihara, A.; Watanabe, Y.; Oshiki, R.; Nishiwaki, T.; Nakamura, K. Association between dietary intake and bone mineral density in Japanese postmenopausal women: The Yokogoshi cohort study. Tohoku J. Exp. Med. 2016, 239, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Choi, K.H.; Park, S.M.; Shin, D.; Joh, H.K.; Cho, E. The benefit of bone health by drinking coffee among Korean postmenopausal women: A cross-sectional analysis of the fourth & fifth Korea national health and nutrition examination surveys. PLoS ONE 2016, 11, e0147762. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Z.H.; Lei, T.; Tang, Z. Subjective evaluation of the frequency of coffee intake and relationship to osteoporosis in Chinese men. J. Health Popul. Nutr. 2016, 35, 24. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.S.; Gallo, M.E.; Saraví, F.D. Yerba Mate (Ilex paraguariensis) consumption is associated with higher bone mineral density in postmenopausal women. Bone 2012, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Pytlik, M.; Zych, M.; Cegieła, U.; Kaczmarczyk-Sedlak, I.; Nowińska, B.; Śliwiński, L. Favorable effect of moderate dose caffeine on the skeletal system in ovariectomized rats. Mol. Nutr. Food Res. 2013, 57, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xue, W.; Liang, S.; Zhao, J.; Zhang, X. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: a systematic review and meta-analysis. Nutr. J. 2016, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, T.; Grant, S.; Yazdani, M. The effects of maternal caffeine intake during pregnancy on mineral contents of fetal rat bone. Res. Exp. Med. 1989, 189, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.E.; Miller, H.I.; Nakamoto, T. Effects of caffeine intake during gestation and lactation on bones of young growing rats. Res. Exp. Med. 1990, 190, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, H.; Cheuk, S.L.; Wink, C.S.; Hashimoto, K.; Rossowska, M.J.; Nakamoto, T. Alteration of femoral structure in later life by chronically feeding caffeine during rapid growing period in newborn female rats. Toxicol. Lett. 1994, 73, 55–64. [Google Scholar] [PubMed]
- Wink, C.S.; Rossowska, M.J.; Nakamoto, T. Effects of caffeine on bone cells and bone development in fast-growing rats. Anat. Rec. 1996, 246, 30–38. [Google Scholar] [CrossRef]
- Ohta, M.; Cheuk, G.; Thomas, K.A.; Kamagata-Kiyoura, Y.; Wink, C.S.; Yazdani, M.; Falster, A.U.; Simmons, W.B.; Nakamoto, T. Effects of caffeine on the bones of aged, ovariectomized rats. Ann. Nutr. Metab. 1999, 43, 52–59. [Google Scholar] [CrossRef]
- Huang, T.H.; Yang, R.S.; Hsieh, S.S.; Liu, S.H. Effects of caffeine and exercise on the development of bone: A densitometric and histomorphometric study in young Wistar rats. Bone 2002, 30, 293–299. [Google Scholar] [CrossRef]
- Ohta, M.; Ide, K.; Cheuk, G.; Cheuk, S.L.; Yazdani, M.; Nakamoto, T.; Thomas, K.A. A caffeine diet can alter the mechanical properties of the bones of young ovariectomized rats. Ann. Nutr. Metab. 2002, 46, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.P.; da Silva, L.R.; de Alvarenga Lemos, V.A.; Duarte, P.M.; Bastos, M.F. Administration of high doses of caffeine increases alveolar bone loss in ligature-induced periodontitis in rats. J. Periodontol. 2008, 79, 2356–2360. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.M.; Marques, M.R.; Bezerra, J.P.; Bastos, M.F. The effects of caffeine administration on the early stage of bone healing and bone density. A histometric study in rats. Arch. Oral Biol. 2009, 54, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chen, C.; Yang, R.S.; Yen, Y.P.; Yang, Y.T.; Tsai, C. Caffeine enhances osteoclast differentiation from bone marrow hematopoietic cells and reduces bone mineral density in growing rats. J. Orthop. Res. 2011, 29, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Olchowik, G.; Chadaj-Polberg, E.; Tomaszewski, M.; Polberg, M.; Tomaszewska, M. The influence of caffeine on the biomechanical properties of bone tissue during pregnancy in a population of rats. Folia Histochem. Cytobiol. 2011, 49, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.; Olchowik, G.; Tomaszewska, M.; Burdan, F. Use of X-ray microprobe to diagnose bone tissue demineralization after caffeine administration. Folia Histochem. Cytobiol. 2012, 50, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.P.; de Siqueira, A.; Pires, A.G.; Marques, M.R.; Duarte, P.M.; Bastos, M.F. Effects of estrogen deficiency and/or caffeine intake on alveolar bone loss, density and healing: A study in rats. J. Periodontol. 2013, 84, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.M.; Brentegani, L.G.; Lacerda, S.A. Effects of coffee intake and intraperitoneal caffeine on bone repair process—A histologic and histometric study. Braz. Dent. J. 2015, 26, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, Y.; Kim, J.; Yu, A.R.; Shin, J.S.; Choi, Y.Y.; Roh, J. High doses of caffeine reduce in vivo osteogenic activity in prepubertal rats. J. Anat. 2015, 227, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, Y.; Kim, J.; Bae, J.; Roh, J. Longitudinal bone growth is impaired by direct involvement of caffeine with chondrocyte differentiation in the growth plate. J. Anat. 2017, 230, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Janas, A.; Pytlik, M.; Cegieła, U.; Śliwiński, L.; Krivošíková, Z.; Štefíková, K.; Gajdoš, M. Effects of trigonelline, an alkaloid present in coffee, on diabetes-induced disorders in the rat skeletal system. Nutrients 2016, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Zych, M.; Nowińska, B.; Pytlik, M.; Janas, A. Unfavorable effect of trigonelline, an alkaloid present in coffee and fenugreek, on bone mechanical properties in estrogen-deficient rats. Mol. Nutr. Food Res. 2014, 58, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Pytlik, M.; Zych, M.; Cegieła, U.; Nowinska, B.; Kaczmarczyk-Sedlak, I.; Sliwinski, L.; Trzeciak, H.; Trzeciak, H.I. Effects of caffeic and chlorogenic acids on the rat skeletal system. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 682–693. [Google Scholar] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Turner, C.H.; Burr, D.B. Basic biomechanical measurements of bone: A tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef]
- Stürmer, E.K.; Seidlová-Wuttke, D.; Sehmisch, S.; Rack, T.; Wille, J.; Frosch, K.H.; Wuttke, W.; Stürmer, K.M. Standardized bending and breaking test for the normal and osteoporotic metaphyseal tibias of the rat: Effect of estradiol, testosterone, and raloxifene. J. Bone Miner. Res. 2006, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Janiec, W.; Barej, M.; Cegieła, U.; Pytlik, M.; Kaczmarczyk-Sedlak, I. Effects of nadroparin on bone histomorphometric parameters in rats. Pol. J. Pharmacol. 2004, 56, 337–343. [Google Scholar] [PubMed]
- Bhatti, S.K.; O’Keefe, J.H.; Lavie, C.J. Coffee and tea: Perks for health and longevity? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Brodt, M.D.; Lynch, M.A.; McKenzie, J.A.; Tanouye, K.M.; Nyman, J.S.; Wang, X. Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J. Bone Miner. Res. 2009, 24, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.; Gürgül, S.; Demirel, C.; Yildiz, A. The effect of insulin therapy on biomechanical deterioration of bone in streptozotocin (STZ)-induced type 1 diabetes mellitus in rats. Diabetes Res. Clin. Pract. 2012, 97, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Coe, L.M.; Zhang, J.; McCabe, L.R. Both spontaneous Ins2(+/−) and streptozotocin-induced type I diabetes cause bone loss in young mice. J. Cell. Physiol. 2013, 228, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Zywert, A.; Szkudelska, K. Metabolic disturbances and defects in insulin secretion in rats with streptozotocin-nicotinamide-induced diabetes. Physiol. Res. 2013, 62, 663–670. [Google Scholar] [PubMed]
- Glajchen, N.; Ismail, F.; Epstein, S.; Jowell, P.S.; Fallon, M. The effect of chronic caffeine administration on serum markers of bone mineral metabolism and bone histomorphometry in the rat. Calcif. Tissue Int. 1988, 43, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, W.; Nishihira, J.; Fujie, K.; Iizuka, T.; Handa, H.; Ozaki, M.; Yukawa, S. Effect of coffee consumption on bone metabolism. Bone 2001, 28, 332–336. [Google Scholar] [CrossRef]
- Brun, L.R.; Brance, M.L.; Lombarte, M.; Maher, M.C.; Di Loreto, V.E.; Rigalli, A. Effects of yerba mate (IIex paraguariensis) on histomorphometry, biomechanics, and densitometry on bones in the rat. Calcif. Tissue Int. 2015, 97, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Nevitt, M.C.; Browner, W.S.; Stone, K.; Fox, K.M.; Ensrud, K.E.; Cauley, J.; Black, D.; Vogt, T.M. Risk factors for hip fracture in white women. Study of osteoporotic fractures research group. N. Engl. J. Med. 1995, 332, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Wolk, A.; Glynn, A.; Michaëlsson, K. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos. Int. 2006, 17, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Bergmann, P.; Boonen, S.; Boutsen, Y.; Bruyere, O.; Devogelaer, J.P.; Goemaere, S.; Hollevoet, N.; Kaufman, J.M.; Milisen, K.; et al. Non-pharmacological management of osteoporosis: A consensus of the Belgian bone club. Osteoporos. Int. 2011, 22, 2769–2788. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yao, K.; Zhang, W.; Zhou, J.; Wu, T.; He, C. Coffee consumption and risk of fractures: A meta-analysis. Arch. Med. Sci. 2012, 8, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Lee, J.; Rota, M.; Lee, J.; Ahn, H.S.; Park, S.M.; Shin, D. Coffee consumption and risk of fractures: A systematic review and dose-response meta-analysis. Bone 2014, 63, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.S. Light coffee consumption is protective against sarcopenia, but frequent coffee consumption is associated with obesity in Korean adults. Nutr. Res. 2017, 41, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W. Caffeine analogs: Biomedical impact. Cell. Mol. Life Sci. 2007, 64, 2153–2169. [Google Scholar] [PubMed]
- Müller, C.E.; Jacobson, K.A. Xanthines as adenosine receptor antagonists. Handb. Exp. Pharmacol. 2011, 200, 151–199. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Folwarczna, J.; Sedlak, L.; Zych, M.; Wojnar, W.; Szumińska, I.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E. Effects of caffeine on the biomarkers of oxidative stress in lenses of rats with streptozotocin-induced diabetes. Arch. Med. Sci. 2017, in press. [Google Scholar]
- Fredholm, B.B.; Yang, J.; Wang, Y. Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Aspects Med. 2017, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Su, S.J.; Chang, K.L.; Su, S.H.; Yeh, Y.T.; Shyu, H.W.; Chen, K.M. Caffeine regulates osteogenic differentiation and mineralization of primary adipose-derived stem cells and a bone marrow stromal cell line. Int. J. Food Sci. Nutr. 2013, 64, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, B.; Abraham, A.A.; Ham, J.; Evans, B.A. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J. Bone Miner. Res. 2011, 26, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Barbosa, A.; Neto, E.; Sá-e-Sousa, A.; Freitas, R.; Neves, J.M.; Magalhães-Cardoso, T.; Ferreirinha, F.; Correia-de-Sá, P. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J. Cell Physiol. 2011, 226, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.H.; Wigner, N.A.; Kulkarni, N.; Johnston-Cox, H.; Gerstenfeld, L.C.; Ravid, K. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J. Biol. Chem. 2012, 287, 15718–15727. [Google Scholar] [CrossRef] [PubMed]
- Kara, F.M.; Doty, S.B.; Boskey, A.; Goldring, S.; Zaidi, M.; Fredholm, B.B.; Cronstein, B.N. Adenosine A1 receptors regulate bone resorption in mice: Adenosine A1 receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A1 receptor-knockout mice. Arthritis Rheum. 2010, 62, 534–541. [Google Scholar] [CrossRef] [PubMed]

| Parameter/Group | Control | STZ | STZ + Caffeine | NA/STZ | NA/STZ + Caffeine |
|---|---|---|---|---|---|
| Osteocalcin (ng/mL) | 243.6 ± 11.3 | 149.9 ± 7.9 * | 167.4 ± 50.9 | 287.4 ± 38.4 | 320.1 ± 26.3 * |
| C-terminal type I collagen fragments (RatLaps) (ng/mL) | 11.29 ± 0.66 | 49.56 ± 11.32 ** | 45.29 ± 5.57 * | 13.22 ± 1.01 | 14.33 ± 1.35 |
| Total calcium (mg/100 mL) | 9.88 ± 0.09 | 8.95 ± 0.40 | 9.80 ± 0.20 | 10.03 ± 0.17 | 9.94 ± 0.24 |
| Fructosamine (mmol/L) | 0.549 ± 0.012 | 1.232 ± 0.117 *** | 1.184 ± 0.075 *** | 0.563 ± 0.020 | 0.528 ± 0.021 |
| Total cholesterol (mg/100 mL) | 36.32 ± 2.28 | 40.80 ± 3.61 | 41.95 ± 1.48 | 34.05 ± 2.43 | 34.65 ± 3.54 |
| Parameter/Group | Control | STZ | STZ + Caffeine | NA/STZ | NA/STZ + Caffeine | |
|---|---|---|---|---|---|---|
| BMC (g) | tibia | 0.261 ± 0.005 | 0.212 ± 0.007 *** | 0.224 ± 0.006 *** | 0.249 ± 0.005 | 0.262 ± 0.008 |
| L-4 vertebra | 0.092 ± 0.002 | 0.069 ± 0.003 *** | 0.071 ± 0.003 *** | 0.087 ± 0.003 | 0.094 ± 0.004 | |
| Bone area (cm2) | tibia | 2.163 ± 0.023 | 2.068 ± 0.029 | 2.084 ± 0.025 | 2.139 ± 0.019 | 2.132 ± 0.025 |
| L-4 vertebra | 0.668 ± 0.014 | 0.644 ± 0.016 | 0.622 ± 0.019 | 0.672 ± 0.012 | 0.690 ± 0.022 | |
| BMD (g/cm2) | tibia | 0.122 ± 0.002 | 0.103 ± 0.003 *** | 0.107 ± 0.003 *** | 0.117 ± 0.002 | 0.123 ± 0.003 |
| L-4 vertebra | 0.137 ± 0.002 | 0.108 ± 0.003 *** | 0.115 ± 0.004 *** | 0.128 ± 0.003 * | 0.135 ± 0.003 | |
| Parameter/Group | Control | STZ | STZ + Caffeine | NA/STZ | NA/STZ + Caffeine | |
|---|---|---|---|---|---|---|
| Tibia | Young’s modulus (MPa) | 3299 ± 287 | 2971 ± 340 | 2340 ± 306 * | 3366 ± 252 | 3704 ± 241 |
| Maximum load (N) | 125.2 ± 7.8 | 54.5 ± 4.6 *** | 50.8 ± 3.9 *** | 103.4 ± 4.8 * | 109.5 ± 6.5 | |
| Displacement for maximum load (mm) | 0.780 ± 0.030 | 0.604 ± 0.042 | 0.677 ± 0.062 | 0.726 ± 0.035 | 0.819 ± 0.077 | |
| Energy for maximum load (mJ) | 48.18 ± 4.65 | 18.34 ± 2.63 *** | 21.21 ± 2.34 *** | 39.02 ± 3.44 | 46.10 ± 6.17 | |
| Stress for maximum load (MPa) | 112.4 ± 8.5 | 57.4 ± 5.9 *** | 48.5 ± 4.1 *** | 96.3 ± 4.6 | 105.4 ± 8.6 | |
| Femur | Diaphysis—maximum load (N) | 121.3 ± 3.8 | 114.4 ± 3.0 | 117.5 ± 3.0 | 118.6 ± 3.9 | 122.2 ± 4.5 |
| Neck—maximum load (N) | 80.7 ± 1.9 | 81.7 ± 2.6 | 79.8 ± 3.5 | 77.8 ± 3.1 | 82.6 ± 3.9 | |
| Parameter/Group | Control | STZ | STZ + Caffeine | NA/STZ | NA/STZ + Caffeine | |
|---|---|---|---|---|---|---|
| Tibia | Transverse cross-sectional area of the cortical bone (mm2) | 3.403 ± 0.074 | 3.138 ± 0.105 | 3.235 ± 0.115 | 3.413 ± 0.072 | 3.379 ± 0.083 |
| Transverse cross-sectional area of the marrow cavity (mm2) | 0.654 ± 0.026 | 0.757 ± 0.045 | 0.851 ± 0.072 | 0.714 ± 0.027 | 0.707 ± 0.034 | |
| Transverse cross-section of the marrow cavity/diaphysis area ratio | 0.162 ± 0.007 | 0.193 ± 0.004 ** | 0.207 ± 0.013 ** | 0.173 ± 0.006 | 0.173 ± 0.008 | |
| Femur | Width of epiphyseal trabeculae (μm) | 56.95 ± 1.33 | 52.90 ± 1.44 | 53.24 ± 0.80 | 53.27 ± 1.29 | 55.72 ± 0.97 |
| Width of metaphyseal trabeculae (μm) | 34.92 ± 1.00 | 31.29 ± 0.86 | 32.58 ± 0.93 | 32.60 ± 1.79 | 32.90 ± 0.55 | |
| With of epiphyseal cartilage (μm) | 46.94 ± 2.82 | 31.10 ± 2.33 ** | 34.79 ± 1.94 ** | 55.64 ± 14.49 | 51.76 ± 3.94 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folwarczna, J.; Janas, A.; Cegieła, U.; Pytlik, M.; Śliwiński, L.; Matejczyk, M.; Nowacka, A.; Rudy, K.; Krivošíková, Z.; Štefíková, K.; et al. Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes. Nutrients 2017, 9, 1196. https://doi.org/10.3390/nu9111196
Folwarczna J, Janas A, Cegieła U, Pytlik M, Śliwiński L, Matejczyk M, Nowacka A, Rudy K, Krivošíková Z, Štefíková K, et al. Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes. Nutrients. 2017; 9(11):1196. https://doi.org/10.3390/nu9111196
Chicago/Turabian StyleFolwarczna, Joanna, Aleksandra Janas, Urszula Cegieła, Maria Pytlik, Leszek Śliwiński, Magdalena Matejczyk, Anna Nowacka, Karolina Rudy, Zora Krivošíková, Kornélia Štefíková, and et al. 2017. "Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes" Nutrients 9, no. 11: 1196. https://doi.org/10.3390/nu9111196




